Sertoli cell
A Sertoli cell (a kind of sustentacular cell) is a "nurse" cell of the testicles that is part of a seminiferous tubule and helps in the process of spermatogenesis, the production of sperm.
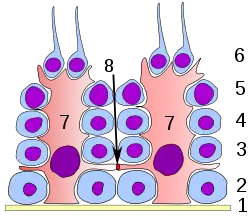 Germinal epithelium of the testicle. 1: basal lamina 2: spermatogonia 3: spermatocyte 1st order 4: spermatocyte 2nd order 5: spermatid 6: mature spermatid 7: Sertoli cell 8: tight junction (blood testis barrier) | |
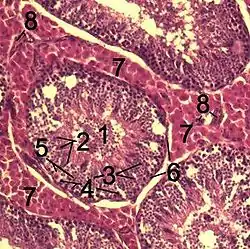 Histological section through testicular parenchyma of a boar. 1 Lumen of Tubulus seminiferus contortus 2 spermatids 3 spermatocytes 4 spermatogonia 5 Sertoli cell 6 Myofibroblasts 7 Leydig cells 8 capillaries | |
| Details | |
|---|---|
| System | Reproductive system |
| Location | Testes |
| Function | Assist in the production of sperm |
| Identifiers | |
| MeSH | D012708 |
| FMA | 72298 |
| Anatomical terms of microanatomy | |
It is activated by follicle-stimulating hormone (FSH) secreted by the adenohypophysis, and has FSH receptor on its membranes. It is specifically located in the convoluted seminiferous tubules (since this is the only place in the testes where the spermatozoa are produced). Development of Sertoli cells is directed by the protein.
Structure
Sertoli cells are located in seminiferous tubules.
On slides, using standard staining, Sertoli cells can easily be confused with the other cells of the germinal epithelium. The most distinctive feature of the Sertoli cell is the dark nucleolus.[1]
Development
Sertoli cells are required for male sexual development. It activates and forms a feedforward loop with FGF9. Sertoli cell proliferation and differentiation is mainly activated by FGF9.[2] [3]
Once fully differentiated, the Sertoli cell has been considered to be terminally differentiated, and is unable to proliferate.[4] Therefore, once spermatogenesis has begun, no more Sertoli cells are created.
Recently however, some scientists have found a way to induce Sertoli cells to a juvenile proliferative phenotype outside of the body.[5] This gives rise to the possibility of repairing some defects that cause male infertility.
It has been suggested that Sertoli cells may derive from the fetal mesonephros.[6]
Function
Because its main function is to nourish the developing sperm cells through the stages of spermatogenesis, the Sertoli cell has also been called the "mother" or "nurse" cell.[7] Sertoli cells also act as phagocytes, consuming the residual cytoplasm during spermatogenesis. Translocation of cells from the base to the lumen of the seminiferous tubules occurs by conformational changes in the lateral margins of the Sertoli cells.
Secretory
Sertoli cells secrete the following substances:
- anti-Müllerian hormone (AMH) — secreted during the early stages of fetal life.
- inhibin and activins — secreted after puberty, and work together to regulate FSH secretion.
- androgen binding protein (also called testosterone binding globulin) — increases testosterone concentration in the seminiferous tubules to lightly stimulate spermatogenesis.
- estradiol — aromatase from Sertoli cells convert testosterone to 17 beta estradiol to direct spermatogenesis
- ETS Related Molecule or ERM transcription factor ERM transcription factor — needed for maintenance of the spermatogonial stem cell in the adult testis.
- transferrin — a blood plasma protein for iron ion delivery[8]
- Testicular ceruloplasmin — a ceruloplasmin-like protein which is immunologically similar to serum ceruloplasmin[9]
Structural
The occluding junctions of Sertoli cells form the blood-testis barrier, a structure that partitions the interstitial blood compartment of the testis from the adluminal compartment of the seminiferous tubules. Because of the apical progression of the spermatogonia (sperm stem cells), the occluding junctions must be dynamically reformed and broken to allow the immunoidentical spermatogonia to cross through the blood-testis barrier so they can become immunologically unique. Sertoli cells control the entry and exit of nutrients, hormones and other chemicals into the tubules of the testis as well as make the adluminal compartment an immune-privileged site.
The cell is also responsible for establishing and maintaining the spermatogonial stem cell niche, which ensures the renewal of stem cells and the differentiation of spermatogonia into mature germ that progress stepwise through the long process of spermatogenesis, ending in the release of spermatozoa in a process known as spermiation.[10] Sertoli cells bind to spermatogonial cells via N-cadherins and galactosyltransferase (via carbohydrate residues).
Other functions
During spermatogenesis sertoli cells provide nutrition to the spermatogonia.
DNA repair and mutation
Sertoli cells are capable of repairing DNA damage.[11] This repair likely employs the process of non-homologous end joining involving XRCC1 and PARP1 proteins that are expressed in Sertoli cells.[11]
Sertoli cells have a higher mutation frequency than spermatogenic cells.[12] Compared to spermatocytes, the mutation frequency is about 5 to 10-fold higher in Sertoli cells. This may reflect the need for greater efficiency of DNA repair and mutation avoidance in the germ line than in somatic cells.
Immunomodulatory properties of Sertoli cells
Besides expressing factors that are crucial for sperm cell maturation, Sertoli cells are producing a wide range of molecules (either on their surface or soluble) that are able to modify the Immune system (IS). The ability of Sertoli cells to change the immune response in the tubule is needed for successful sperm cell maturation. Sperm cells are expressing neoepitopes on their surface as they progress through different stages of maturation. They can trigger a strong immune response if placed in a different site of the body.
Molecules produced by Sertoli cells associated with immunosuppression or immunoregulation
FAS/FAS-L system – expression of Fas ligand (Fas-L) on the surface of SCs activates apoptotic death of Fas receptor bearing cells, f.e. cytotoxic T-cells.[13]
- soluble FasL- increasing the effectivity of the system
- soluble Fas- FasL blockage on the surface of other cells ( no apoptotic induction in Sertoli cells by cells of IS)
B7/H1 – decreasing proliferation of effector T-cells[14]
Jagged1 (JAG1) – induction of Foxp3 transcription factor expression in naive T lymphocytes (increasing relative numbers of T regulatory cells)[15]
Protease inhibitor-9 (PI-9) – member of serpin family (serine protease inhibitors)[16]
- induces secretion of protease Granyzme B, cytotoxic T-cells and NK cells are able to induce apoptosis in target cell. SCs produce PI-9 that irreversibly bonds Granzyme B and inhibits its activity
CD59 - surface molecule on SCs, member of the Complement Regulatory Proteins (CRP)
- inhibits the last step of complement cascade – formation of the Membrane Atack Complex[17]
Clusterin – a soluble molecule, function similar to CD59 – making complex with Granyzme B and inhibits activation of apoptosis by T-lymphocytes or NK cells[17]
TGF-beta – transforming growth factor beta (its direct production by SCs is controversial)
- induction of regulatory T-cells in periphery[18]
Another molecules involved
CD40 - molecule associated with Dendritic Cells (DC)
- SCs are able to down regulate the expression of CD40 on the surface of DCs (mechanism not known)
- Downregulation of CD40 results in decreased ability of DCs to stimulate the T-cell response[17]
Sertoli cells are also able to inhibit the migration of immune cells – lower immune cells infiltration to the site of inflammation.
Clinical significance
Sertoli-Leydig cell tumour is part of the sex cord-stromal tumour group of ovarian neoplasms. These tumors produce both Sertoli and Leydig cells and lead to an increased secretion of testosterone in ovaries and testicles.
Other animals
Function of Sertoli cells in amniota and anamniota is the same, but they have a slightly different properties when compared to each other. Anamnionts (fish and amphibians) are employing cystic spermatogenesis in order to produce sperm cells.[19] In case of amniota Sertoli cells are considered to be terminally differentiated cells not able to proliferate. In anamniota Sertoli cells go through two proliferative phases. First phase of proliferation occurs during cyst establishment promoting also migration of germ cells into it.[20][21] second one is to enlarge the cyst and produce a space for proliferating germ cells.[22]
Commonly accepted fact that Sertol cells are terminally differentiated in amniota was recently changed. After xenogeneic transplantation Sertoli cells were able to proliferate.[23]
History
Sertoli cells are called so because of their eponym Enrico Sertoli, an Italian physiologist who discovered them while studying medicine in the University of Pavia, Italy.[24]
He published a description of this cell in 1865. The cell was discovered by Sertoli with a Belthle microscope purchased in 1862, which he used while studying medicine.
In the 1865 publication, his first description used the terms "tree-like cell" or "stringy cell" and most importantly he referred to these as "mother cells". It was other scientists who used Enrico's family name, Sertoli, to label these cell in publications, starting in 1888. As of 2006, two textbooks that are devoted specifically to the Sertoli cell have been published.
Research
Recently (2016), experimental models of autoimmune inflammatory disorders, including diabetes, have prompted the implication of Sertoli cells into cell therapy transplantation thanks to their immunoregulatory and anti-inflammatory properties.[25]
Research adopting Sertoli cells in Diabetes type I. treatment is in the deepest stage by now. The strategy is to cotransplant β cells together with Sertoli cells into recipient organism. In case of mice, rats and also human presence of these cells restored glucose homeostasis together with lower requirement of external insulin. In all cases no immunosuppression was used, the role of this medication was taken and provided by SC.[26][27][28]
By treating spontaneously diabetic and obese mice with the transplantation of microencapsulated Sertoli cells in the subcutaneous abdominal fat depot, Giovanni et al.[25] have demonstrated that more than the half of the treated mice showed improved glucose homeostasis. This recent scientific work promises a future better treatment to patients with type 2 diabetes mellitus through the use of cell therapy.
Sertoli cells promote skin graft acceptation by recipient organism[29] and also their presence helps to increase numbers of motor neurons in spinal cord of SOD1 mice (mouse model of Amyotrofic lateral sclerosis)[30]
Additional images
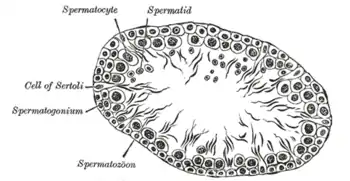 section of a tubule of the testis of a rat. X 250.
section of a tubule of the testis of a rat. X 250.
References
- OSU Center for Veterinary Health Sciences - OSU-CVHS Home Archived 2006-12-09 at the Wayback Machine
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B (June 2006). "Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination". PLOS Biology. 4 (6): e187. doi:10.1371/journal.pbio.0040187. PMC 1463023. PMID 16700629.
- Moniot B, Declosmenil F, Barrionuevo F, Scherer G, Aritake K, Malki S, Marzi L, Cohen-Solal A, Georg I, Klattig J, Englert C, Kim Y, Capel B, Eguchi N, Urade Y, Boizet-Bonhoure B, Poulat F (June 2009). "The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation". Development. 136 (11): 1813–21. doi:10.1242/dev.032631. PMC 4075598. PMID 19429785.
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS (June 2003). "Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood". Reproduction. 125 (6): 769–84. doi:10.1530/reprod/125.6.769. PMID 12773099.
- Nicholls PK, Stanton PG, Chen JL, Olcorn JS, Haverfield JT, Qian H, Walton KL, Gregorevic P, Harrison CA (December 2012). "Activin signaling regulates Sertoli cell differentiation and function". Endocrinology. 153 (12): 6065–77. doi:10.1210/en.2012-1821. PMID 23117933.
- Vize PD, Woolf AS, Bard J (2003). The kidney: from normal development to congenital disease. Academic Press. pp. 82–. ISBN 978-0-12-722441-1. Retrieved 18 November 2010.
- Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, Oliveira PF (May 2012). "Metabolic regulation is important for spermatogenesis". Nature Reviews. Urology. 9 (6): 330–8. doi:10.1038/nrurol.2012.77. PMID 22549313. S2CID 7385545.
- Xiong X, Wang A, Liu G, Liu H, Wang C, Xia T, Chen X, Yang K (July 2006). "Effects of p,p'-dichlorodiphenyldichloroethylene on the expressions of transferrin and androgen-binding protein in rat Sertoli cells". Environmental Research. 101 (3): 334–9. Bibcode:2006ER....101..334X. doi:10.1016/j.envres.2005.11.003. PMID 16380112.
- Skinner MK, Griswold MD (June 1983). "Sertoli cells synthesize and secrete a ceruloplasmin-like protein". Biology of Reproduction. 28 (5): 1225–1229. doi:10.1095/biolreprod28.5.1225. PMID 6871315.
- O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG (January 2011). "Spermiation: The process of sperm release". Spermatogenesis. 1 (1): 14–35. doi:10.4161/spmg.1.1.14525. PMC 3158646. PMID 21866274.
- Ahmed EA, Barten-van Rijbroek AD, Kal HB, Sadri-Ardekani H, Mizrak SC, van Pelt AM, de Rooij DG (June 2009). "Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells". Biology of Reproduction. 80 (6): 1084–91. doi:10.1095/biolreprod.108.071662. PMID 19164176.
- Walter CA, Intano GW, McCarrey JR, McMahan CA, Walter RB (August 1998). "Mutation frequency declines during spermatogenesis in young mice but increases in old mice". Proceedings of the National Academy of Sciences of the United States of America. 95 (17): 10015–9. Bibcode:1998PNAS...9510015W. doi:10.1073/pnas.95.17.10015. PMC 21453. PMID 9707592.
- Dal Secco V, Riccioli A, Padula F, Ziparo E, Filippini A (February 2008). "Mouse Sertoli cells display phenotypical and functional traits of antigen-presenting cells in response to interferon gamma". Biology of Reproduction. 78 (2): 234–42. doi:10.1095/biolreprod.107.063578. PMID 17989360.
- Kaur G, Thompson LA, Dufour JM (June 2014). "Sertoli cells--immunological sentinels of spermatogenesis". Seminars in Cell & Developmental Biology. 30: 36–44. doi:10.1016/j.semcdb.2014.02.011. PMC 4043859. PMID 24603046.
- Campese AF, Grazioli P, de Cesaris P, Riccioli A, Bellavia D, Pelullo M, Padula F, Noce C, Verkhovskaia S, Filippini A, Latella G, Screpanti I, Ziparo E, Starace D (March 2014). "Mouse Sertoli cells sustain de novo generation of regulatory T cells by triggering the notch pathway through soluble JAGGED1". Biology of Reproduction. 90 (3): 53. doi:10.1095/biolreprod.113.113803. PMID 24478388.
- Potempa J, Korzus E, Travis J (June 1994). "The serpin superfamily of proteinase inhibitors: structure, function, and regulation". The Journal of Biological Chemistry. 269 (23): 15957–60. doi:10.1016/S0021-9258(17)33954-6. PMID 8206889.
- Lee HM, Oh BC, Lim DP, Lee DS, Lim HG, Park CS, Lee JR (June 2008). "Mechanism of humoral and cellular immune modulation provided by porcine sertoli cells". Journal of Korean Medical Science. 23 (3): 514–20. doi:10.3346/jkms.2008.23.3.514. PMC 2526533. PMID 18583891.
- Iliadou PK, Tsametis C, Kaprara A, Papadimas I, Goulis DG (October 2015). "The Sertoli cell: Novel clinical potentiality". Hormones. 14 (4): 504–14. doi:10.14310/horm.2002.1648. PMID 26859601.
- Schulz RW, de França LR, Lareyre JJ, Le Gac F, LeGac F, Chiarini-Garcia H, Nobrega RH, Miura T (February 2010). "Spermatogenesis in fish". General and Comparative Endocrinology. 165 (3): 390–411. doi:10.1016/j.ygcen.2009.02.013. PMID 19348807.
- Morais RD, Nóbrega RH, Gómez-González NE, Schmidt R, Bogerd J, França LR, Schulz RW (November 2013). "Thyroid hormone stimulates the proliferation of Sertoli cells and single type A spermatogonia in adult zebrafish (Danio rerio) testis". Endocrinology. 154 (11): 4365–76. doi:10.1210/en.2013-1308. PMID 24002037.
- Lacerda SM, Costa GM, Campos-Junior PH, Segatelli TM, Yazawa R, Takeuchi Y, Morita T, Yoshizaki G, França LR (February 2013). "Germ cell transplantation as a potential biotechnological approach to fish reproduction". Fish Physiology and Biochemistry. 39 (1): 3–11. doi:10.1007/s10695-012-9606-4. PMID 22290474. S2CID 10134737.
- Almeida FF, Kristoffersen C, Taranger GL, Schulz RW (January 2008). "Spermatogenesis in Atlantic cod (Gadus morhua): a novel model of cystic germ cell development". Biology of Reproduction. 78 (1): 27–34. doi:10.1095/biolreprod.107.063669. PMID 17881768.
- Mital P, Kaur G, Bowlin B, Paniagua NJ, Korbutt GS, Dufour JM (January 2014). "Nondividing, postpubertal rat sertoli cells resumed proliferation after transplantation". Biology of Reproduction. 90 (1): 13. doi:10.1095/biolreprod.113.110197. PMC 4076399. PMID 24285718.
- synd/518 at Who Named It?
- Luca G, Arato I, Mancuso F, Calvitti M, Falabella G, Murdolo G, Basta G, Cameron DF, Hansen BC, Fallarino F, Baroni T, Aglietti MC, Tortoioli C, Bodo M, Calafiore R (November 2016). "Xenograft of microencapsulated Sertoli cells restores glucose homeostasis in db/db mice with spontaneous diabetes mellitus". Xenotransplantation. 23 (6): 429–439. doi:10.1111/xen.12274. PMID 27678013. S2CID 46744082.
- Valdés-González RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Mendez AJ, Dávila-Pérez R, Elliott RB, Terán L, White DJ (September 2005). "Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study". European Journal of Endocrinology. 153 (3): 419–27. doi:10.1530/eje.1.01982. PMID 16131605.
- Korbutt GS, Elliott JF, Rajotte RV (February 1997). "Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression". Diabetes. 46 (2): 317–22. doi:10.2337/diab.46.2.317. PMID 9000711.
- Li Y, Xue W, Liu H, Fan P, Wang X, Ding X, Tian X, Feng X, Pan X, Zheng J, Tian P, Ding C, Fan X (2013-02-20). "Combined strategy of endothelial cells coating, Sertoli cells coculture and infusion improves vascularization and rejection protection of islet graft". PLOS ONE. 8 (2): e56696. Bibcode:2013PLoSO...856696L. doi:10.1371/journal.pone.0056696. PMC 3577699. PMID 23437215.
- Bistoni G, Calvitti M, Mancuso F, Arato I, Falabella G, Cucchia R, Fallarino F, Becchetti A, Baroni T, Mazzitelli S, Nastruzzi C, Bodo M, Becchetti E, Cameron DF, Luca G, Calafiore R (July 2012). "Prolongation of skin allograft survival in rats by the transplantation of microencapsulated xenogeneic neonatal porcine Sertoli cells". Biomaterials. 33 (21): 5333–40. doi:10.1016/j.biomaterials.2012.04.020. PMID 22560198.
- Hemendinger, Richelle; Wang, Jay; Malik, Saafan; Persinski, Rafal; Copeland, Jane; Emerich, Dwaine; Gores, Paul; Halberstadt, Craig; Rosenfeld, Jeffrey (2005). "Sertoli cells improve survival of motor neurons in SOD1 transgenic mice, a model of amyotrophic lateral sclerosis". Experimental Neurology. 196 (2): 235–243. doi:10.1016/j.expneurol.2005.07.025. PMID 16242126. S2CID 352751.
External links
- Histology image: 17805loa – Histology Learning System at Boston University
- Histology image: 17806loa – Histology Learning System at Boston University