Tight junction
Tight junctions, also known as occluding junctions or zonulae occludentes (singular, zonula occludens) are multiprotein junctional complexes whose canonical function is to prevent leakage of solutes and water and seals between the epithelial cells. Tight junctions may also serve as leaky pathways by forming selective channels for small cations, anions, or water. Tight junctions are present mostly in vertebrates (with the exception of Tunicates[1]). The corresponding junctions that occur in invertebrates are septate junctions.
| Tight junction | |
|---|---|
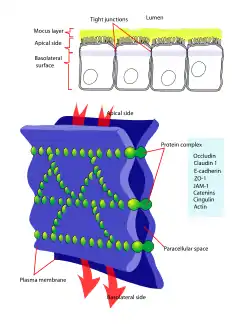 Diagram of Tight junction | |
| Details | |
| Identifiers | |
| Latin | junctio occludens |
| MeSH | D019108 |
| TH | H1.00.01.1.02007 |
| FMA | 67397 |
| Anatomical terminology | |
Structure
Tight junctions are composed of a branching network of sealing strands, each strand acting independently from the others. Therefore, the efficiency of the junction in preventing ion passage increases exponentially with the number of strands. Each strand is formed from a row of transmembrane proteins embedded in both plasma membranes, with extracellular domains joining one another directly. There are at least 40 different proteins composing the tight junctions.[2] These proteins consist of both transmembrane and cytoplasmic proteins. The three major transmembrane proteins are occludin, claudins, and junction adhesion molecule (JAM) proteins. These associate with different peripheral membrane proteins such as ZO-1 located on the intracellular side of plasma membrane, which anchor the strands to the actin component of the cytoskeleton.[3] Thus, tight junctions join together the cytoskeletons of adjacent cells.
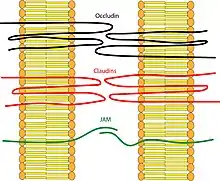
Transmembrane proteins:
- Occludin was the first integral membrane protein to be identified. It has a molecular weight of ~60kDa. It consists of four transmembrane domains and both the N-terminus and the C-terminus of the protein are intracellular. It forms two extracellular loops and one intracellular loop. These loops help regulate paracellular permeability.[4] Occludin also plays a key role in cellular structure and barrier function.[5]
- Claudins were discovered after occludin and are a family of over 27 different members in mammals.[6] They have a molecular weight of ~20kDa. They have a structure similar to that of occludin in that they have four transmembrane domains and similar loop structure. They are understood to be the backbone of tight junctions and play a significant role in the tight junction's ability to seal the paracellular space.[7]
- Junctional Adhesion Molecules (JAM) are part of the immunoglobulin superfamily. They have a molecular weight of ~40kDa. Their structure differs from that of the other integral membrane proteins in that they only have one transmembrane domain instead of four. It helps to regulate the paracellular pathway function of tight junctions and is also involved in helping to maintain cell polarity.[8]
- Angulins were discovered in 2011 by visual screening of proteins which localize at tricellular tight junctions.[9] There are three members of angulins, Angulin-1/LSR, Angulin-2/ILDR1, and Angulin-3/ILDR2. Similar to JAMs, angulins are single-transmembrane proteins. All angulins have one immunoglobulin-like domain in the extracellular region and one PDZ-binding motif at the carboxy-terminus. They are responsible for establishment of tricellular tight junctions and regulate the paracellular barrier function.[10]
Functions
They perform vital functions:[11]
- Tight junctions provide endothelial and epithelial cells with barrier function, which can be further subdivided into protective barriers and functional barriers serving purposes such as material transport and maintenance of osmotic balance:
- Tight junctions prevent the passage of molecules and ions through the space between plasma membranes of adjacent cells, so materials must actually enter the cells (by diffusion or active transport) in order to pass through the tissue. Investigation using freeze-fracture methods in electron microscopy is ideal for revealing the lateral extent of tight junctions in cell membranes and has been useful in showing how tight junctions are formed.[12] The constrained intracellular pathway exacted by the tight junction barrier system allows precise control over which substances can pass through a particular tissue. (Tight junctions play this role in maintaining the blood–brain barrier.) At the present time, it is still unclear whether the control is active or passive and how these pathways are formed. In one study for paracellular transport across the tight junction in kidney proximal tubule, a dual pathway model is proposed: large slit breaks formed by infrequent discontinuities in the TJ complex and numerous small circular pores.[13]
- Tight junctions help to maintain the apicobasal polarity of cells by preventing the lateral diffusion of integral membrane proteins between the apical and lateral/basal surfaces, allowing the specialized functions of each surface (for example receptor-mediated endocytosis at the apical surface and exocytosis at the basolateral surface) to be preserved. This allows polarized transcellular transport and specialised functions of apical and basolateral membranes.
Classification
Epithelia are classed as "tight" or "leaky", depending on the ability of the tight junctions to prevent water and solute movement:[14]
- Tight epithelia have tight junctions that prevent most movement between cells. Examples of tight epithelia include the distal convoluted tubule, the collecting duct of the nephron in the kidney, and the bile ducts ramifying through liver tissue. Other examples are the blood-brain barrier and the blood cerebrospinal fluid barrier
- Leaky epithelia do not have these tight junctions, or have less complex tight junctions. For instance, the tight junction in the kidney proximal tubule, a very leaky epithelium, has only two to three junctional strands, and these strands exhibit infrequent large slit breaks.
See also
- Cadherin
- Gap junction
- Tight junction protein (disambiguation)
- Zonulin

References
- Banerjee, Swati; Sousa, Aurea D.; Bhat, Manzoor A. (2006). "Organization and Function of Septate Junctions: An Evolutionary Perspective". Cell Biochemistry and Biophysics. 46 (1): 65–78. doi:10.1385/CBB:46:1:65. ISSN 1085-9195. PMID 16943624. S2CID 3119021.
- Itallie, Christina M. Van; Anderson, James M. (2009-08-01). "Physiology and Function of the Tight Junction". Cold Spring Harbor Perspectives in Biology. 1 (2): a002584. doi:10.1101/cshperspect.a002584. ISSN 1943-0264. PMC 2742087. PMID 20066090.
- Anderson, JM; Van Itallie, CM (August 2009). "Physiology and function of the tight junction". Cold Spring Harb Perspect Biol. 1 (2): a002584. doi:10.1101/cshperspect.a002584. PMC 2742087. PMID 20066090.
- Wolburg, Hartwig; Lippoldt, Andrea; Ebnet, Klaus (2006), "Tight Junctions and the Blood-Brain Barrier", Tight Junctions, Springer US, pp. 175–195, doi:10.1007/0-387-36673-3_13, ISBN 9780387332017
- Liu, Wei-Ye; Wang, Zhi-Bin; Zhang, Li-Chao; Wei, Xin; Li, Ling (2012-06-12). "Tight Junction in Blood-Brain Barrier: An Overview of Structure, Regulation, and Regulator Substances". CNS Neuroscience & Therapeutics. 18 (8): 609–615. doi:10.1111/j.1755-5949.2012.00340.x. ISSN 1755-5930. PMC 6493516. PMID 22686334.
- Schneeberger, Eveline E.; Lynch, Robert D. (June 2004). "The tight junction: a multifunctional complex" (PDF). American Journal of Physiology. Cell Physiology. 286 (6): C1213–C1228. doi:10.1152/ajpcell.00558.2003. ISSN 0363-6143. PMID 15151915. S2CID 1725292. Archived from the original (PDF) on 2019-02-22.
- Mitic, Laura L.; Van Itallie, Christina M.; Anderson, James M. (August 2000). "Molecular Physiology and Pathophysiology of Tight Junctions I. Tight junction structure and function: lessons from mutant animals and proteins" (PDF). American Journal of Physiology. Gastrointestinal and Liver Physiology. 279 (2): G250–G254. doi:10.1152/ajpgi.2000.279.2.g250. ISSN 0193-1857. PMID 10915631. S2CID 32634345. Archived from the original (PDF) on 2019-03-09.
- Luissint, Anny-Claude; Artus, Cédric; Glacial, Fabienne; Ganeshamoorthy, Kayathiri; Couraud, Pierre-Olivier (2012-11-09). "Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation". Fluids and Barriers of the CNS. 9 (1): 23. doi:10.1186/2045-8118-9-23. ISSN 2045-8118. PMC 3542074. PMID 23140302.
- Masuda, Sayuri; Oda, Yukako; Sasaki, Hiroyuki; Ikenouchi, Junichi; Higashi, Tomohito; Akashi, Masaya; Nishi, Eiichiro; Furuse, Mikio (2011-02-15). "LSR definescell corners for tricellular tight junction formation in epithelial cells". Journal of Cell Science. 124 (Part 4): 548–555. doi:10.1242/jcs.072058. PMID 21245199.
- Higashi, Tomohito; Miller, Ann (2017-07-15). "Tricellular junctions: how to build junctions at the TRICkiest points of epithelial cells". Molecular Biology of the Cell. 28 (15): 2023–2034. doi:10.1091/mbc.E16-10-0697. ISSN 1939-4586. PMC 5509417. PMID 28705832.
- Department, Biology. "Tight Junctions (and other cellular connections)". Davidson College. Retrieved 2015-01-12.
- Chalcroft, J. P.; Bullivant, S (1970). "An interpretation of liver cell membrane and junction structure based on observation of freeze-fracture replicas of both sides of the fracture". The Journal of Cell Biology. 47 (1): 49–60. doi:10.1083/jcb.47.1.49. PMC 2108397. PMID 4935338.
- Guo, P; Weinstein, AM; Weinbaum, S (Aug 2003). "A dual-pathway ultrastructural model for the tight junction of rat proximal tubule epithelium" (PDF). American Journal of Physiology. Renal Physiology. 285 (2): F241–57. doi:10.1152/ajprenal.00331.2002. PMID 12670832. S2CID 22824832. Archived from the original (PDF) on 2019-02-22.
- Department, Biology. "Tight Junctions and other cellular connections". Davidson College. Retrieved 2013-09-20.
External links
- An Overview of the Tight Junction at Zonapse.Net
- Occludin in Focus at Zonapse.Net
- Tight+Junctions at the US National Library of Medicine Medical Subject Headings (MeSH)
- Histology image: 20502loa – Histology Learning System at Boston University