Glans penis
The glans penis, commonly referred to as the glans, is a bulbous structure at the distal end of the human penis that is the most sensitive erogenous zone and primary anatomical source of male sexual pleasure.[2][3] It is anatomically homologous to the clitoral glans. The glans penis is part of the male reproductive system in humans and other mammals where it may appear smooth, spiny, elongated or divided.[4] It is externally lined with mucosal tissue, which creates a smooth texture and glossy appearance. The glans is completely or partially covered by the foreskin/prepuce in humans, except for those born without one or who had it cut off. The foreskin can generally be retracted over and past the glans, and may automatically retract during an erection. The glans is more commonly known as the "head" or the "tip" of the penis, and colloquially referred to in British English as the “bellend”. The medical name comes from the Latin words glans ("acorn") and penis ("of the penis").
| Glans penis | |
|---|---|
 Human glans penis (dorsal view) | |
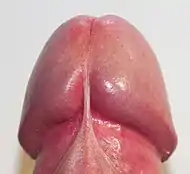 Glans penis (ventral view) | |
| Details | |
| Synonyms | Glans |
| Pronunciation | /ɡlænz/[1] |
| Precursor | Genital tubercle |
| System | Urogenital system |
| Artery | Dorsal artery |
| Vein | Deep dorsal vein |
| Nerve | Dorsal nerve |
| Identifiers | |
| Latin | Glans penis |
| TA98 | A09.4.01.007 |
| TA2 | 3668 |
| FMA | 18247 |
| Anatomical terminology | |
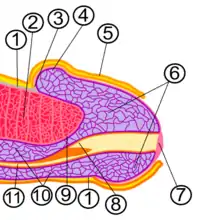
- Fascia penis
- Corpus cavernosum
- Coronal sulcus
- Corona of glans
- Foreskin
- Glans penis
- Meatus of the urethra
- Navicular fossa of male urethra
- Tunica albuginea of penis
- Corpus spongiosum
- Urethra
In humans
Structure
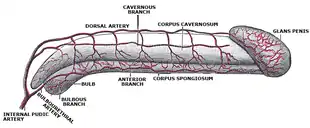
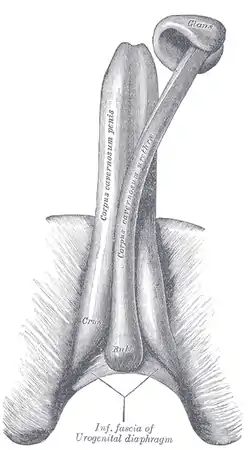
The glans penis is a body of spongy erectile tissue that is moulded on the rounded ends of the two corpora cavernosa penis,[5] extending farther on their upper than on their lower surfaces. It is the expanded cap of the corpus spongiosum,[6] a sponge-like region that surrounds the male urethra within the penis maintaining it as a viable channel for ejaculation.[7] Externally it is lined with mucosal tissue, which is responsible for its smooth texture and appearance. The increase of arterial flow during erection fills the erectile tissue with blood causing the glans to grow in size and sensitivity.[8] While the penis is rigid when erect, the glans itself remains slightly softer.[9] The soft cushiony texture of the glans absorbs impact during rigorous instances of copulation.[10] The proportional size of the glans penis can vary greatly. While the shape of the glans is typically acorn-like, in some men it might be much wider in circumference than the shaft, giving the penis a mushroom-like appearance, while in others it might be narrower and more akin to a probe in shape.[9] Some researchers have suggested that the glans has evolved to become acorn-, mushroom- or cone-shaped so that during copulation it acts to remove any semen still there from previous sex partners, but this is not supported when looking at primate relatives who have different mating behaviors.[11][12]
At the summit of the glans is the slit-like vertical external urethral orifice, called the urinary meatus, through which urine, semen and pre-ejaculatory fluid exit the penis. The circumference of the base of the glans forms a rounded projecting border, the corona glandis, overhanging a deep retroglandular groove known as the coronal sulcus, behind which is the neck of the penis.[9] The frenulum is the highly vascularized elastic band of tissue located on the underside of the glans that connects the foreskin to the head of the penis. The frenulum is supple enough to allow the retraction of the foreskin over the glans and pull it back when the erection is gone.[13] In flaccid state, it tightens to narrow the foreskin opening.[14]
Innervation
The glans and the frenulum are innervated by the dorsal nerve of the penis and the perineal nerve, both divisions of the pudendal nerve.[15] Branches of the dorsal nerve extend through the glans ventrolaterally displaying a more three-dimensional innervation pattern.[15][16] The main branches form smaller bundles of nerves that expand outwards into the tissue of the glans.[15] The rich innervation of the glans penis reveals its function as primary anatomical source of male sexual pleasure.[3][16] Yang & Bradley argue; "the distinct pattern of innervation of the glans emphasizes the role of the glans as a sensory structure".[3] While Yang & Bradley's (1998) report "showed no areas in the glans to be more densely innervated than others.",[3] Halata & Munger (1986) report that the density of several nerve terminals is greatest in the corona glandis.[17]
Halata & Spathe (1997) reported; "the glans penis contains a predominance of free nerve endings, numerous genital end bulbs and rarely Pacinian and Ruffinian corpuscles. Merkel nerve endings and Meissner's corpuscles (mechanoreceptors typically found in thick glabrous skin) are not present."[18] The genital end bulbs, that are present throughout the glans, are most numerous in the corona and near the frenulum.[19] Pacinian and Ruffini corpuscles are identified predominantly in the corona glandis. The most numerous nerve terminals are free nerve endings present in almost every dermal papilla of the glans, as well as scattered throughout the deeper dermis.[19]
Vascularization
The glans penis receives blood from the internal pudental artery through its branch, the dorsal artery of the penis that runs along the penile shaft.[20] Behind the corona, the terminal branches of the dorsal arteries anastomose with the axial arteries through perforating branches before they end in the glans.[21] Venous drainage of the penis begins at the base of the glans. Smaller tributaries deriving from the corona form a venous plexus at the neck of the penis, known as the retro-coronal plexus.[22] The deep dorsal vein, one of the two dorsal veins of the penis, serves as a common vessel receiving blood drained from the glans and the corpora cavernosa through the circumflex veins that surround them.[23][22]
Foreskin
Typically, the glans is completely or partially covered by a double-layered fold of skin, known as the foreskin. Glans exposure can be easily achieved by manual retraction of the foreskin or sometimes automatically during erection. The degree of automatic foreskin retraction varies considerably depending on the foreskin length.[24] The primary purpose of the foreskin is considered to be the covering of the glans and the urinary meatus,[25] while also maintaining the mucosa in a moist environment.[27]
Foreskin rectractability gradually increases with age. In infancy the foreskin is fused to the glans,[28] it remains non retractable in early childhood and it is generally tight during preadolescence.[29] The skin begins to loosen up significantly during puberty allowing the glans to be completely exposed when needed. By the age of eighteen most boys will have a fully retractable foreskin.[30]
In some cases, for medical, religious or cultural reasons some men might undergo circumcision, a procedure where the foreskin is partially or completely removed from the penis.[14] The glans of circumcised men remains permanently exposed and dry. Several studies have suggested the glans is generally equally sensitive in both circumcised and uncircumcised penises.[31][32][33]
Development
The glans develops from a phallic structure, called the genital tubercle, which forms in the embryo regardless of sex during the early weeks of pregnancy.[34] Initially undifferentiated, the tubercle develops into a penis during the development of the reproductive system depending on the exposure to male hormones, such as androgens.
In mammals, sexual differentiation is determined by the sperm that carries either an X or a Y (male) chromosome.[35] The Y chromosome contains a sex-determining gene (SRY) that encodes a transcription factor for the protein TDF (testis determining factor) and triggers the creation of testosterone for the embryo's development into a male.[36][37]
Although the sex of the infant is determined from the moment of conception,[34] the complete external differentiation of the organs begins about eight or nine weeks after conception.[38] Some sources state that the process will be completed by the twelfth week,[39][40] while others state that it is clearly evident by the thirteenth week and that the sex organs are fully developed by the sixteenth week.[36] Both penis and clitoris develop from the same tissues that become the glans and shaft of the penis and this shared embryonic origin makes these two organs homologous (different versions of the same structure).[41][36]
In the female fetus the absence of testosterone will stop the growth of the phallus causing the tubercle to shrink and form the clitoris. In the male fetus the presence of a Y chromosome leads to the development of the testes, which secrete a large amount of hormones called androgens. These hormones will cause the masculinization of the phenotypically indifferent organs.[34] When exposed to testosterone, the genital tubercle elongates to form the penis. By fusion of the urogenital folds – elongated spindle-shaped structures that contribute to the formation of the urethral groove on the belly aspect of the genital tubercle – the urogenital sinus closes completely to form the spongy urethra and the labioscrotal swellings unite to form the scrotum.[42][36] The secretion of testosterone during this phase plays a decisive role in the final shaping of the penis. After birth, testosterone levels drop significantly until puberty.
Clinical significance
The epithelium of the glans penis consists of mucosal tissue. Birley et al. report that excessive washing with soap may dry the mucous membrane which covers the glans penis and cause non-specific dermatitis. The condition is described as an inflammation of the skin, often caused by an irritating substance or a contact allergy. Sensitivity to chemicals in certain products can cause an allergic reaction, including irritation, itching and rash.[43]
Inflammation of the glans penis is known as balanitis. It is a treatable condition that occurs in about 3–11% of males (up to 35% of diabetic males). Edwards reported that it is generally more common in males who have poor hygiene habits or have not been circumcised. It has many causes, including irritation or infection with a wide variety of pathogens. Symptoms of balanitis may appear suddenly or develop gradually. They might include pain, irritation, redness or red patches on the glans penis. Careful identification of the cause with the aid of patient history, physical examination, swabs and cultures, and biopsy are essential in order to determine the proper treatment.[44]
The meatus (opening) of the urethra located at the tip of the glans might become subject to meatal stenosis, a condition mostly seen as a late complication of circumcision. It occurs in about 2–20% of circumcised boys[45][46] and it is rarely seen in uncircumcised men.[47] It is characterized by a narrowing of the meatus, which might cause sudden or often urges to urinate and burning during the process.[47]
Other animals
Male felids are able to urinate backwards by curving the tip of the glans penis backward.[48][49] In cats, the glans penis is covered with spines, while in dogs the glans is smooth. Penile spines also occur on the glans of male and female spotted hyenas.[48] In male dogs, the glans penis consists of two parts called the bulbus glandis and pars longa glandis.[50] The glans of a fossa's penis extends about halfway down the shaft and is spiny except at the tip. In comparison, the glans of felids is short and spiny, while that of viverrids is smooth and long.[51] The shape of the glans varies among different marsupial species.[52][53][54] In most marsupials, the glans is divided, but male macropods have an undivided glans penis.[4] The glans penis is also divided into two parts in platypuses and echidnas.[55][56]
The glans penis of the marsh rice rat is long and robust,[57] averaging 7.3 mm (0.29 in) long and 4.6 mm (0.18 in) broad.[58]
In Thomasomys ucucha the glans penis is rounded, short, and small and is superficially divided into left and right halves by a trough at the top and a ridge at the bottom. Most of the glans is covered with spines, except for an area near the tip.[59]
Winkelmann's mouse can most readily be distinguished from its close relatives by its partially corrugated glans penis.[60]
When erect, the glans of a horse's penis increases by 3 to 4 times. The urethra opens within the urethral fossa, a small pouch at the distal end of the glans.[61] Unlike the human glans, the glans of a horse's penis extends backwards on its shaft.[62][63][64][65][66][67][68][69][70]
Males of Racey's pipistrelle bat have a narrow, egg-shaped glans penis.[71]
The glans penis of a male cape ground squirrel is large with a prominent baculum.[72]
References
- OED 2nd edition, 1989.
- Olausson, Håkan; Wessberg, Johan; Morrison, India (2016). Affective Touch and the Neurophysiology of CT Afferents. Springer Science+Business Media. p. 305. ISBN 978-1-4939-6418-5.
...the most pleasurable of all body parts when stimulated sexually: the glans (or tip) of the penis.
- Yang, C. C.; W.E. Bradley (July 1998). "Neuroanatomy of the penile portion of the human dorsal nerve of the penis". British Journal of Urology. 82 (1): 109–13. doi:10.1046/j.1464-410x.1998.00669.x. PMID 9698671.
- Renfree, Marilyn; Hugh Tyndale-Biscoe (1987-01-30). Reproductive Physiology of Marsupials. Cambridge University Press. ISBN 978-0-521-33792-2. Retrieved 5 May 2013.
- Heide Schatten; Gheorghe M. Constantinescu (21 March 2008). Comparative Reproductive Biology. John Wiley & Sons. ISBN 978-0-470-39025-2.
- Lee, Shin-Hyo; Ha, Tae-Jun; Koh, Ki-Seok; Song, Wu-Chul (2019). "Ligamentous structures in human glans penis". Journal of Anatomy. 234 (1): 83–88. doi:10.1111/joa.12896. PMC 6284436. PMID 30450557.
- "penis | Description, Anatomy, & Physiology | Britannica". www.britannica.com. Retrieved 2022-09-24.
- Dean, Robert C.; Lue, Tom F. (2005–2011). "Physiology of Penile Erection and Pathophysiology of Erectile Dysfunction". The Urologic Clinics of North America. 32 (4): 379–v. doi:10.1016/j.ucl.2005.08.007. ISSN 0094-0143. PMC 1351051. PMID 16291031.
- "Glans Penis: Anatomy, Function, and Common Conditions". Healthline. 2020-05-08. Retrieved 2022-09-24.
- HSU, G‐L., et al. "The distribution of elastic fibrous elements within the human penis." BJU International 73.5 (1994): 566-571.
- Gallup, Gordon G., et al. "The human penis as a semen displacement device." Evolution and Human Behavior 24.4 (2003): 277-289
- Dixson, Alan F. (2009). Sexual Selection and the Origins of Human Mating Systems. OUP Oxford. p. 68. ISBN 978-0-19-156973-9.
- "Penis Frenulum: Location, Function & Conditions". Cleveland Clinic. Retrieved 2022-09-24.
- "Male circumcision: Global trends and determinants of prevalence, safety and acceptability" (PDF). World Health Organization. 2007. Archived (PDF) from the original on 2015-07-15. Retrieved 2009-06-12.
- Weech, David; Ameer, Muhammad Atif; Ashurst, John V. (2022), "Anatomy, Abdomen and Pelvis, Penis Dorsal Nerve", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30247841, retrieved 2022-09-26
- Yang, Claire C.; Bradley, William E. (1999-01-01). "Innervation of the human glans penis". Journal of Urology. 161 (1): 97–102. doi:10.1016/S0022-5347(01)62075-5. PMID 10037378.
- Halata, Zdenek; Bryce L. Munger (April 1986). "The neuroanatomical basis for the protopathic sensibility of the human glans penis". Brain Research. 371 (2): 205–30. doi:10.1016/0006-8993(86)90357-4. PMID 3697758. S2CID 23781274.
- Halata, Zdenek; A. Spaethe (1997). Sensory innervation of the human penis. Advances in Experimental Medicine and Biology. Vol. 424. pp. 265–6. doi:10.1007/978-1-4615-5913-9_48. ISBN 978-0-306-45696-1. PMID 9361804. Retrieved 2006-07-07.
- Halata, Z.; Munger, B. L. (1986-04-23). "The neuroanatomical basis for the protopathic sensibility of the human glans penis". Brain Research. 371 (2): 205–230. doi:10.1016/0006-8993(86)90357-4. ISSN 0006-8993. PMID 3697758. S2CID 23781274.
- Clement, Pierre; Giuliano, Francois (2015). "3 - Anatomy and physiology of genital organs – men". Handbook of Clinical Neurology. Vol. 130. Elsevier. pp. 19–37. doi:10.1016/B978-0-444-63247-0.00003-1. ISBN 978-0-444-63247-0. ISSN 0072-9752. PMID 26003237.
- Quartey, J. K.M. (2006), Schreiter, F.; Jordan, G.H. (eds.), "Anatomy and Blood Supply of the Urethra and Penis", Urethral Reconstructive Surgery, Berlin, Heidelberg: Springer, p. 14, doi:10.1007/3-540-29385-x_3, ISBN 978-3-540-29385-9, retrieved 2022-10-29
- Quartey, J. K.M. (2006), Schreiter, F.; Jordan, G.H. (eds.), "Anatomy and Blood Supply of the Urethra and Penis", Urethral Reconstructive Surgery, Berlin, Heidelberg: Springer, p. 16, doi:10.1007/3-540-29385-x_3, ISBN 978-3-540-29385-9, retrieved 2022-10-29
- Hsu, Geng-Long; Hsieh, Cheng-Hsing; Wen, Hsien-Sheng; Chen, Yi-Chang; Chen, Shyh-Chyan; Mok, Martin S. (2003-11-12). "Penile Venous Anatomy: An Additional Description and Its Clinical Implication". Journal of Andrology. 24 (6): 921–927. doi:10.1002/j.1939-4640.2003.tb03145.x.
- Velazquez, Elsa F.; Bock, Adelaida; Soskin, Ana; Codas, Ricardo; Arbo, Manuel; Cubilla, Antonio L. (2003). "Preputial variability and preferential association of long phimotic foreskins with penile cancer: an anatomic comparative study of types of foreskin in a general population and cancer patients". The American Journal of Surgical Pathology. 27 (7): 994–998. doi:10.1097/00000478-200307000-00015. ISSN 0147-5185. PMID 12826892.
- Kirby R, Carson C, Kirby M (2009). Men's Health (3rd ed.). New York: Informa Healthcare. p. 283. ISBN 978-1-4398-0807-8. OCLC 314774041.
- Cold, C. J.; Taylor, J. R. (1999). "The prepuce". BJU International. 83 (S1): 34–44. doi:10.1046/j.1464-410x.1999.0830s1034.x. ISSN 1464-410X.
- Dave, Sumit; Afshar, Kourosh; Braga, Luis H.; Anderson, Peter (2018). "CUA guideline on the care of the normal foreskin and neonatal circumcision in Canadian infants". Canadian Urological Association Journal. 12 (2). doi:10.5489/cuaj.5033. ISSN 1920-1214.
At birth, the inner foreskin is usually fused to the glans penis and should not be forcibly retracted
- Dave, Sumit; Afshar, Kourosh; Braga, Luis H.; Anderson, Peter (2018). "CUA guideline on the care of the normal foreskin and neonatal circumcision in Canadian infants". Canadian Urological Association Journal. 12 (2). doi:10.5489/cuaj.5033. ISSN 1920-1214.
the incidence of non- retractable physiological phimosis was 50% in grade 1 boys and decreased to 35% in grade 4 and 8% in grade 7 boys
- McGregor, Thomas B.; Pike, John G.; Leonard, Michael P. (2007). "Pathologic and physiologic phimosis: Approach to the phimotic foreskin". Canadian Family Physician. 53 (3).
most foreskins will become retractile by adulthood.
- Bleustein, Clifford B.; James D. Fogarty; Haftan Eckholdt; Joseph C. Arezzo; Arnold Melman (April 2005). "Effect of neonatal circumcision on penile neurologic sensation". Urology. 65 (4): 773–7. doi:10.1016/j.urology.2004.11.007. PMID 15833526.
- Bleustein, Clifford B.; Haftan Eckholdt; Joseph C. Arezzo; Arnold Melman (April 26 – May 1, 2003). "Effects of Circumcision on Male Penile Sensitivity". American Urological Association 98th Annual Meeting. Chicago, Illinois. Archived from the original on February 7, 2005.
- Payne, Kimberley; Thaler, Lea; Kukkonen, Tuuli; Carrier, Serge; Binik, Yitzchak (May 2007). "Sensation and Sexual Arousal in Circumcised and Uncircumcised Men". Journal of Sexual Medicine. 4 (3): 667–674. doi:10.1111/j.1743-6109.2007.00471.x. PMID 17419812.
- W.George, D.Wilson, Fredrick, Jean (1984). "Fetal Physiology and Medicine (Second, Revised Edition)". ScienceDirect.
- Arulkumaran, Sabaratnam; Regan, Lesley; Papageorghiou, Aris; Monga, Ash; Farquharson, David (2011-06-23). Oxford Desk Reference: Obstetrics and Gynaecology. OUP Oxford. ISBN 978-0-19-162087-4.
- Schünke, Michael; Schulte, Erik; Lamperti, Edward D.; Schumacher, Udo (2006). Thieme Atlas of Anatomy: General Anatomy and Musculoskeletal System. Thieme. ISBN 978-1-58890-387-7.
- "Genetic Mechanisms of Sex Determination | Learn Science at Scitable". www.nature.com. Retrieved 2022-09-23.
- Merz, Eberhard; Bahlmann, F. (2004). Ultrasound in Obstetrics and Gynecology. Vol. 1. Thieme Medical Publishers. ISBN 978-1-58890-147-7.
- C.L.Lachelin, Gillian (1991). "Introduction to Clinical Reproductive Endocrinology". ScienceDirect.
- Merz, Eberhard; Bahlmann, F. (2004). Ultrasound in Obstetrics and Gynecology. Vol. 1. Thieme Medical Publishers. ISBN 978-1-58890-147-7
- Sloane, Ethel (2002). Biology of Women. Delmar Thomson Learning. ISBN 978-0-7668-1142-3.
- Sloane, Ethel (2002). Biology of Women. Cengage Learning. ISBN 978-0-7668-1142-3. Archived from the original on 13 June 2013. Retrieved 27 October 2015.
- "Contact Dermatitis: Irritants, Allergies, Symptoms & Treatment". Cleveland Clinic. Retrieved 2022-09-25.
- "Balanitis: Types, Symptoms, Causes, Treatments, Prevention & Relief". Cleveland Clinic. Retrieved 2022-09-25.
- Sorokan SK, Finlay JC, Jefferies AL (2015). "Newborn male circumcision". Paediatrics & Child Health. 20 (6): 311–320. doi:10.1093/pch/20.6.311. PMC 4578472. PMID 26435672. Retrieved 20 October 2017.
- Koenig JF (22 September 2016). "Meatal stenosis". EMedicine. Retrieved 2 October 2017.
- "Meatal Stenosis: Symptoms, Diagnosis & Treatment - Urology Care Foundation". www.urologyhealth.org. Retrieved 2022-09-25.
- R. F. Ewer (1973). The Carnivores. Cornell University Press. pp. 116–. ISBN 978-0-8014-8493-3. Retrieved 8 February 2013.
- Reena Mathur (2010). Animal Behaviour 3/e. Rastogi Publications. ISBN 978-81-7133-747-7. Retrieved 10 February 2013.
- Howard E. Evans; Alexander de Lahunta (7 August 2013). Miller's Anatomy of the Dog. Elsevier Health Sciences. ISBN 978-0-323-26623-9.
- Köhncke, M.; Leonhardt, K. (1986). "Cryptoprocta ferox" (PDF). Mammalian Species (254): 1–5. doi:10.2307/3503919. JSTOR 3503919. Retrieved 19 May 2010.
- Australian Mammal Society (December 1978). Australian Mammal Society. Australian Mammal Society. pp. 73–. Retrieved 25 December 2012.
- Wilfred Hudson Osgood; Charles Judson Herrick (1921). A monographic study of the American marsupial, Caēnolestes …. University of Chicago. pp. 64–. Retrieved 25 December 2012.
- The Urologic and Cutaneous Review. Urologic & Cutaneous Press. 1920. pp. 677–. Retrieved 25 December 2012.
- Mervyn Griffiths (2 December 2012). The Biology of the Monotremes. Elsevier Science. ISBN 978-0-323-15331-7.
- Libbie Henrietta Hyman (15 September 1992). Hyman's Comparative Vertebrate Anatomy. University of Chicago Press. ISBN 978-0-226-87013-7.
- Hooper and Musser, 1964, p. 13
- Hooper and Musser, 1964, table 1
- Voss, 2003, p. 11
- Bradley, R.D.; Schmidley, D.J. (1987). "The glans penes and bacula in Latin American taxa of the Peromyscus boylii group". Journal of Mammalogy. 68 (3): 595–615. doi:10.2307/1381595. JSTOR 1381595.
- "The Stallion: Breeding Soundness Examination & Reproductive Anatomy". University of Wisconsin-Madison. Archived from the original on 2007-07-16. Retrieved 7 July 2007.
- Mating Males: An Evolutionary Perspective on Mammalian Reproduction. Cambridge University Press. 30 June 2012. ISBN 978-1-107-00001-8. Retrieved 5 May 2013.
- Bassert, Joanna M; McCurnin, Dennis M (2013-04-01). McCurnin's Clinical Textbook for Veterinary Technicians - Joanna M Bassert, Dennis M McCurnin - Google Boeken. ISBN 978-1-4557-2884-8. Retrieved 2013-08-16.
- Research, Equine (2005-07-01). Horseman's Veterinary Encyclopedia, Revised and Updated - Equine Research - Google Boeken. ISBN 978-0-7627-9451-5. Retrieved 2013-08-16.
- Weese, Scott; Graham Munroe, Dr; Munroe, Graham (2011-03-15). Equine Clinical Medicine, Surgery and Reproduction - Graham Munroe BVSc (Hons) PhD Cert EO DESM Dip ECVS FRCVS, Scott Weese DVM DVSc DipACVIM - Google Boeken. ISBN 978-1-84076-608-0. Retrieved 2013-08-16.
- König, Horst Erich; Hans-Georg, Hans-Georg; Bragulla, H (2007). Veterinary Anatomy of Domestic Mammals: Textbook and Colour Atlas - Google Boeken. ISBN 978-3-7945-2485-3. Retrieved 2013-08-16.
- Hedge, Juliet (2004). Horse Conformation: Structure, Soundness, and Performance - Equine Research - Google Boeken. ISBN 978-1-59228-487-0. Retrieved 2013-08-16.
- Evans, Warren J; Borton, Anthony; Hintz, Harold; Dale Van Vleck, L (1990-02-15). The Horse - Google Boeken. ISBN 978-0-7167-1811-6. Retrieved 2013-08-16.
- Schatten, Heide; Constantinescu, Gheorghe M (2008-03-21). Comparative Reproductive Biology - Heide Schatten, Gheorghe M. Constantinescu - Google Boeken. ISBN 978-0-470-39025-2. Retrieved 2013-08-16.
- McKinnon, Angus O; Squires, Edward L; Vaala, Wendy E; Varner, Dickson D (2011-07-05). Equine Reproduction - Google Boeken. ISBN 978-0-470-96187-2. Retrieved 2013-08-16.
- Bates et al., 2006, pp. 306–307
- Skurski, D., J. Waterman. 2005. "Xerus inauris", Mammalian Species 781:1-4.