Beta wave
Beta waves, or beta rhythm, are a neural oscillation (brainwave) in the brain with a frequency range of between 12.5 and 30 Hz (12.5 to 30 cycles per second). Beta waves can be split into three sections: Low Beta Waves (12.5–16 Hz, "Beta 1"); Beta Waves (16.5–20 Hz, "Beta 2"); and High Beta Waves (20.5–28 Hz, "Beta 3").[1] Beta states are the states associated with normal waking consciousness.
History
Beta waves were discovered and named by the German psychiatrist Hans Berger, who invented electroencephalography (EEG) in 1924, as a method of recording electrical brain activity from the human scalp. Berger termed the larger amplitude, slower frequency waves that appeared over the posterior scalp when the subject's eye were closed alpha waves. The smaller amplitude, faster frequency waves that replaced alpha waves when the subject opened their eyes were then termed beta waves.[2]
Function
Low-amplitude beta waves with multiple and varying frequencies are often associated with active, busy or anxious thinking and active concentration.[3]
Over the motor cortex, beta waves are associated with the muscle contractions that happen in isotonic movements and are suppressed prior to and during movement changes.[4] Bursts of beta activity are associated with a strengthening of sensory feedback in static motor control and reduced when there is movement change.[5] Beta activity is increased when movement has to be resisted or voluntarily suppressed.[6] The artificial induction of increased beta waves over the motor cortex by a form of electrical stimulation called Transcranial alternating-current stimulation consistent with its link to isotonic contraction produces a slowing of motor movements.[7]
Investigations of reward feedback have revealed two distinct beta components; a high beta (low gamma) component,[8] and low beta component.[9] In association with unexpected gains, the high beta component is more profound when receiving an unexpected outcome, with a low probability.[10] However the low beta component is said to be related to the omission of gains, when gains are expected.[9]
Relationship with GABA
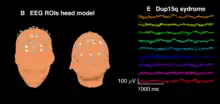
Beta waves are often considered indicative of inhibitory cortical transmission mediated by gamma aminobutyric acid (GABA), the principal inhibitory neurotransmitter of the mammalian nervous system. Benzodiazepines, drugs that modulate GABAA receptors, induce beta waves in EEG recordings from humans [11] and rats.[12] Spontaneous beta waves are also observed diffusely in scalp EEG recordings from children with duplication 15q11.2-q13.1 syndrome (Dup15q) who have duplications of GABAA receptor subunit genes GABRA5, GABRB3, and GABRG3.[13] Similarly, children with Angelman syndrome with deletions of the same GABAA receptor subunit genes feature diminished beta amplitude.[14] Thus, beta waves are likely biomarkers of GABAergic dysfunction, especially in neurodevelopmental disorders caused by 15q deletions/duplications.
Brainwaves
- Delta wave – (0.1 – 3 Hz)
- Theta wave – (4 – 7 Hz)
- Alpha wave – (7 – 12 Hz)
- Beta wave – (12 – 38 Hz)
- Gamma wave – (38 – 100 Hz)
References
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Bauer LO, Rohrbaugh J, O'Connor SJ, Kuperman S, Reich T, Begleiter (2002). "Beta power in the EEG of alcoholics". Biological Psychology. 52 (8): 831–842. doi:10.1016/s0006-3223(02)01362-8. PMID 12372655. S2CID 26052409.
- Buzsáki, György (2006). Rhythms of the Brain. New York: Oxford University Press. p. 4. ISBN 978-0-19-530106-9.
- Baumeister J, Barthel T, Geiss KR, Weiss M (2008). "Influence of phosphatidylserine on cognitive performance and cortical activity after induced stress". Nutritional Neuroscience. 11 (3): 103–110. doi:10.1179/147683008X301478. PMID 18616866. S2CID 45936526.
- Baker, SN (2007). "Oscillatory interactions between sensorimotor cortex and the periphery". Current Opinion in Neurobiology. 17 (6): 649–55. doi:10.1016/j.conb.2008.01.007. PMC 2428102. PMID 18339546.
- Lalo, E; Gilbertson, T; Doyle, L; Di Lazzaro, V; Cioni, B; Brown, P (2007). "Phasic increases in cortical beta activity are associated with alterations in sensory processing in the human". Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale. 177 (1): 137–45. doi:10.1007/s00221-006-0655-8. PMID 16972074. S2CID 24685610.
- Zhang, Y; Chen, Y; Bressler, SL; Ding, M (2008). "Response preparation and inhibition: the role of the cortical sensorimotor beta rhythm". Neuroscience. 156 (1): 238–46. doi:10.1016/j.neuroscience.2008.06.061. PMC 2684699. PMID 18674598.
- Pogosyan, A; Gaynor, LD; Eusebio, A; Brown, P (2009). "Boosting cortical activity at Beta-band frequencies slows movement in humans". Current Biology. 19 (19): 1637–41. doi:10.1016/j.cub.2009.07.074. PMC 2791174. PMID 19800236.
- Marco-Pallerés, J., Cucurell, D., Cunillera, T., García, R., Andrés-Pueyo, A., Münte, T. F., et al. (2008).Human oscillatory activity associated to reward processing in a gambling task, Neuropsychologia, 46, 241-248. doi:10.1016/j.neuropsychologia.2007.07.016
- Yaple, Z., Martinez-Saito, M., Novikov, N., Altukhov, D., Shestakova, A., Klucharev, V. (2018). Power of feedback-induced beta oscillations reflect omission of rewards: Evidence from an EEG gambling study, Frontiers in Neuroscience, 12, 776. doi:10.3389/fnins.2018.00776
- HajiHosseini, A., Rodriguez-Fornells, A., and Marco-Pallerés, J. (2012). The role of beta-gamma oscillations in unexpected rewards processing, Neuroimage, 60, 1678-1685. doi:10.1016/j.neuroimage.2012.01.125
- Feshchenko, V; Veselis, R; Reinsel, R (1997). "Comparison of the EEG effects of midazolam, thiopental, and propofol: the role of underlying oscillatory systems". Neuropsychobiology. 35 (4): 211–20. doi:10.1159/000119347. PMID 9246224.
- Van Lier, Hester; Drinkenburg, Wilhelmus; Van Eeten, Yvonne; Coenen, Anton (2004). "Effects of diazepam and zolpidem on EEG beta frequencies are behavior-specific in rats". Neuropharmacology. 47 (2): 163–174. doi:10.1016/j.neuropharm.2004.03.017. PMID 15223295. S2CID 20725910.
- Frohlich, Joel; Senturk, Damla; Saravanapandian, Vidya; Golshani, Peyman; Reiter, Lawrence; Sankar, Raman; Thibert, Ronald; DiStefano, Charlotte; Huberty, Scott; Cook, Edwin; Jeste, Shafali (December 2016). "A Quantitative Electrophysiological Biomarker of Duplication 15q11.2-q13.1 Syndrome". PLOS ONE. 11 (12): e0167179. Bibcode:2016PLoSO..1167179F. doi:10.1371/journal.pone.0167179. PMC 5157977. PMID 27977700.
- Hipp, Joerg F.; Khwaja, Omar; Krishnan, Michelle; Jeste, Shafali S.; Rotenberg, Alexander; Hernandez, Maria-Clemencia; Tan, Wen-Hann; Sidorov, Michael S.; Philpot, Benjamin D. (2019-01-18). "Electrophysiological Phenotype in Angelman Syndrome Differs Between Genotypes". Biological Psychiatry. 85 (9): 752–759. doi:10.1016/j.biopsych.2019.01.008. ISSN 0006-3223. PMC 6482952. PMID 30826071.