Child-resistant packaging
Child-resistant packaging or CR packaging is special packaging used to reduce the risk of children ingesting hazardous materials. This is often accomplished by the use of a special safety cap. It is required by regulation for prescription drugs, over-the-counter medications, Nicotine Containing Electronic Cigarette devices or Refill containers that can contain Nicotine EUTPD 36.7[1][2][3] pesticides, and household chemicals.[4] In some jurisdictions, unit packaging such as blister packs is also regulated for child safety.[5]
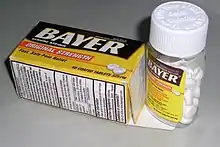
The U.S. Consumer Product Safety Commission has stated in a press release that "There is no such thing as child-proof packaging. So you shouldn't think of packaging as your primary line of defense. Rather, you should think of packaging, even child-resistant packaging, as your last line of defense."[6]
Background
The child-resistant locking closure for containers was invented in 1967 by Dr. Henri Breault.[7]
A history of accidents involving children opening household packaging and ingesting the contents led the United States Congress to pass the Poison Prevention Packaging Act of 1970, authored by U.S. Senator Frank E. Moss of Utah. This gave the U.S. Consumer Product Safety Commission[8][9] the authority to regulate this area. Additions throughout the decades have increased the initial coverage to include other hazardous items, including chemicals regulated by the Environmental Protection Agency. Although child-resistant lids are not perfect, there is strong evidence that use of child-resistant lids has reduced child poisoning rates in the United States substantially.[10] Coordination exists for improving international standards on requirements and protocols.
Difficulty opening
Child-resistant packaging can be a problem for some aged individuals or people with disabilities.[11][12][13] Regulations require designs to be tested to verify that most adults can access the package.[14] Some jurisdictions allow pharmacists to provide medications in non-CR packages when there are no children in the same house.
Requirements
The regulations are based on protocols of performance tests of packages with actual children, to determine if the packages can be opened. More recently, additional package testing is used to determine if aged individuals or people with disabilities have the ability to open the same packages.[15][16]
Often the CR requirements are met by package closures which require two dissimilar motions for opening. Hundreds of package designs are available for packagers to consider.
Standards
- ISO 8317 Child-resistant packaging - Requirements and testing procedures for reclosable packages.
- ISO 13127 Packaging—Child resistant packaging—Mechanical test methods for reclosable child resistant packaging systems
- ASTM D3475, Standard Classification for Child-Resistant Packages
- ASTM F3159, Consumer Safety Specification for Liquid Laundry Packets
- ASTM F2517-17 Standard Specification for Determination of Child Resistance of Portable Fuel Containers for Consumer Use
See also
- Child safety lock
- Childproof
- Package pilferage
- Over-the-counter drug
- Pharmaceutical
- Screw cap
- Tamper resistance
References
- "The Tobacco and Related Products Regulations 2016". www.legislation.gov.uk. Archived from the original on 2017-03-31. Retrieved 2017-03-30.
- Sanbar, Shafeek S. (2007). Legal medicine. Elsevier Health Sciences. p. 393. ISBN 978-0-323-03753-2. Archived from the original on 31 March 2017. Retrieved 6 November 2010.
- Winter, Harold (2005-05-01). Trade-offs: an introduction to economic reasoning and social issues. University of Chicago Press. p. 98. ISBN 978-0-226-90225-8. Archived from the original on 2017-03-31. Retrieved 6 November 2010.
- Gaunt, Michael J. (May 2007). "Child-resistant does not mean Childproof". Pharmacy Times. Retrieved 3 March 2009.
- Smith, G; Barone, S (16 March 2005). "PPPA, Unit Packaging" (PDF). CPSC. Archived from the original (PDF) on 22 July 2010. Retrieved 25 March 2010.
- "New National Emergency Hotline Assessed; CPSC Joins in Launching Poison Prevention Week to Stop 30 Deaths Each Year" (Press release). CPSC. Archived from the original on 12 December 2008. Retrieved 26 March 2009.
- "Dr. Henri Breault". Canadian Medical Hall of Fame. Archived from the original on 7 March 2012. Retrieved 28 February 2012.
- CPSC (February 9, 2007). "Poison Prevention Packaging Information". U.S. Consumer Product Safety Commission. Archived from the original on 19 December 2008. Retrieved 19 December 2008.
- Viscusi, W. Kip (1995). Fatal tradeoffs: public and private responsibilities for risk. Oxford University Press US. p. 235. ISBN 978-0-19-510293-2. Retrieved 6 November 2010.
- Schwebel, D C (2017). "Unintentional child poisoning risk: A review of causal factors and prevention studies" (PDF). Children's Health Care. 46 (2): 109–130. doi:10.1080/02739615.2015.1124775. S2CID 73912457. Archived (PDF) from the original on October 21, 2018. Retrieved October 21, 2018.
- de la Fuente, Javier. "The use of a universal design methodology for developing child-resistant drug packaging". Master's Thesis. Michigan State University. Archived from the original on 2020-10-09. Retrieved 2013-10-16.
- de la Fuente, Javier; Bix, Laura. "Perceptions and attitudes of people with disabilities and older adults about child-resistant drug packaging" (PDF). Journal for Patient Compliance. Archived from the original (PDF) on 2013-10-17. Retrieved 2013-10-16.
- de la Fuente, Javier; Bix, Laura (2010). User-pack interaction: Insights for Designing Inclusive Child-resistant Packaging. Designing Inclusive Interactions. pp. 89–100. doi:10.1007/978-1-84996-166-0_9. ISBN 978-1-84996-165-3.
- CPSC. "Testing procedure for special packaging". U.S. Consumer Product Safety Commission. Retrieved 21 August 2015.
- CPSC. "Poison Prevention Packaging". US Consumer Product Safety Commission. Archived from the original on 5 September 2015. Retrieved 21 August 2015.
- Bix, Laura; de la Fuente, Javier; Pimple, Kenneth D.; Kou, Eric (2009). "Is the test of senior friendly/child resistant packaging ethical?". Health Expectations. 12 (2): 430–437. doi:10.1111/j.1369-7625.2009.00534.x. PMC 5060504. PMID 19650857.
General references
- Yam, K. L., "Encyclopedia of Packaging Technology", John Wiley & Sons, 2009, ISBN 978-0-470-08704-6
- Lockhart, H., and Paine, F.A., "Packaging of Pharmaceuticals and Healthcare Products", 2006, Blackie, ISBN 0-7514-0167-6
External links
- Institute of Packaging Professionals
- Packaging Association of Canada
- Jay M. Arena Papers at Duke University Medical Center Archives (During the 1950s Arena persuaded drug companies to develop childproof safety caps for medicine bottles)
News stories and press releases:
- Mother pushes for childproof packaging at ToledoBlade.com, 2005-08-02
- Childproof Packaging - new designs could save lives at EPSRC, 2005-05-24