Electroconvulsive therapy
Electroconvulsive therapy (ECT) is a psychiatric treatment where a generalized seizure (without muscular convulsions) is electrically induced to manage refractory mental disorders.[1] Typically, 70 to 120 volts are applied externally to the patient's head, resulting in approximately 800 milliamperes of direct current passing between the electrodes, for a duration of 100 milliseconds to 6 seconds, either from temple to temple (bilateral ECT) or from front to back of one side of the head (unilateral ECT). However, only about 1% of the electrical current crosses the bony skull into the brain because skull impedance is about 100 times higher than skin impedance.[2]
| Electroconvulsive therapy | |
|---|---|
 MECTA spECTrum 5000Q with electroencephalography (EEG) in a modern ECT suite | |
| Other names | Electroshock therapy |
| ICD-10-PCS | GZB |
| ICD-9-CM | 94.27 |
| MeSH | D004565 |
| OPS-301 code | 8-630 |
| MedlinePlus | 007474 |
The ECT procedure was first conducted in 1938 by Italian psychiatrist Ugo Cerletti[3] and rapidly replaced less safe and effective forms of biological treatments in use at the time. ECT is often used with informed consent[4] as a safe and effective intervention for major depressive disorder, mania, and catatonia.[5] ECT machines were originally placed in the Class III category by the United States Food and Drug Administration (FDA) in 1976.[6] They were re-classified as Class II devices, for treatment of catatonia, major depressive disorder, and bipolar disorder, in 2018.[7]
Aside from effects on the brain, the general physical risks of ECT are similar to those of brief general anesthesia.[8]: 259 Immediately following treatment, the most common adverse effects are confusion and transient memory loss.[5][9] Among treatments for severely depressed pregnant women, ECT is one of the least harmful to the fetus.[10]
A usual course of ECT involves multiple administrations, typically given two or three times per week until the patient no longer has symptoms. ECT is administered under anesthesia with a muscle relaxant.[11] ECT can differ in its application in three ways: electrode placement, treatment frequency, and the electrical waveform of the stimulus. These treatment parameters can pose significant differences in both adverse side effects and symptom remission in the treated patient.
Placement can be bilateral, where the electric current is passed from one side of the brain to the other, or unilateral, in which the current is solely passed across one hemisphere of the brain. High-dose unilateral ECT has some cognitive advantages compared to moderate-dose bilateral ECT while showing no difference in antidepressant efficacy.[12]
ECT appears to work in the short term via an anticonvulsant effect primarily in the frontal lobes and longer term via neurotrophic effects primarily in the medial temporal lobe.[13]
Medical use
ECT is used, where possible, with informed consent[4] in treatment-resistant major depressive disorder, treatment-resistant catatonia, prolonged or severe mania, and in conditions where "there is a need for rapid, definitive response because of the severity of a psychiatric or medical condition (e.g., when illness is characterized by stupor, marked psychomotor retardation, depressive delusions or hallucinations, or life-threatening physical exhaustion associated with mania)."[5][14] It has also been used to treat autism in adults with an intellectual disability, yet findings from a systematic review found this an unestablished intervention.[15]
Major depressive disorder
For major depressive disorder, despite a Canadian guideline and some experts arguing for using ECT as a first line treatment,[16][17][18] ECT is generally used only when one or other treatments have failed, or in emergencies, such as imminent suicide.[5][19][20] ECT has also been used in selected cases of depression occurring in the setting of multiple sclerosis, Parkinson's disease, Huntington's chorea, developmental delay, brain arteriovenous malformations, and hydrocephalus.[21]
Efficacy
A meta-analysis on the effectiveness of ECT in unipolar and bipolar depression was conducted in 2012. Results indicated that although patients with unipolar depression and bipolar depression responded to other medical treatments very differently, both groups responded equally well to ECT. Overall remission rate for patients given a round of ECT treatment was 50.9% for those with unipolar depression and 53.2% for those with bipolar depression. The severity of each patient's depression was assessed at the same baseline in each group. Most severely depressed patients respond to ECT.[22]
In 2004, a meta-analytic review paper found in terms of efficacy, "a significant superiority of ECT in all comparisons: ECT versus simulated ECT, ECT versus placebo, ECT versus antidepressants in general, ECT versus tricyclics and ECT versus monoamine oxidase inhibitors."[23]
In 2003, The UK ECT Review Group published a systematic review and meta-analysis comparing ECT to placebo and antidepressant drugs. This meta-analysis demonstrated a large effect size (high efficacy relative to the mean in terms of the standard deviation) for ECT versus placebo, and versus antidepressant drugs.[24]
Compared with repetitive transcranial magnetic stimulation (rTMS) for people with treatment-resistant major depressive disorder, ECT relieves depression as shown by reducing the score on the Hamilton Rating Scale for Depression by about 15 points, while rTMS reduced it by 9 points.[25]
The response rate is from 50 to 60% in treatment-resistant patients.[26] Efficacity does not depend on depression subtype.[17]
Follow-up
There is little agreement on the most appropriate follow-up to ECT for people with major depressive disorder.[27] When ECT is followed by treatment with antidepressants, about 50% of people relapsed by 12 months following successful initial treatment with ECT, with about 37% relapsing within the first 6 months. About twice as many relapsed with no antidepressants. Most of the evidence for continuation therapy is with tricyclic antidepressants; evidence for relapse prevention with newer antidepressants is lacking.[27]
Lithium has also been found to reduce the risk of relapse; especially in younger patients.[28]
Catatonia
ECT is generally a second-line treatment for people with catatonia who do not respond to other treatments, but is a first-line treatment for severe or life-threatening catatonia.[5][29][30] There is a plethora of evidence for its efficacy, notwithstanding a lack of randomised controlled trials, such that "the excellent efficacy of ECT in catatonia is generally acknowledged".[29] For people with autism spectrum disorders who have catatonia, there is little published evidence about the efficacy of ECT; as of 2014 there were twelve case reports.[31]
Mania
ECT is used to treat people who have severe or prolonged mania;[5] NICE recommends it only in life-threatening situations or when other treatments have failed[32] and as a second-line treatment for bipolar mania.[33][34]
Schizophrenia
ECT is widely used worldwide in the treatment of schizophrenia, but in North America and Western Europe it is invariably used only in treatment resistant schizophrenia when symptoms show little response to antipsychotics; there is comprehensive research evidence for such practice.[35] It is useful in the case of severe exacerbations of catatonic schizophrenia, whether excited or stuporous.[5][32] There are also case reports of ECT improving persistent psychotic symptoms associated with stimulant-induced psychosis.[36][37]
Effects
Aside from effects in the brain, the general physical risks of ECT are similar to those of brief general anesthesia; the US Surgeon General's report says that there are "no absolute health contraindications" to its use.[8]: 259 Immediately following treatment, the most common adverse effects are confusion and memory loss. Some patients experience muscle soreness after ECT. A meta-analysis from 2017 found that the death rate of ECT is around 2.1 per 100,000 procedures.[38] There is evidence and rationale to support giving low doses of benzodiazepines or otherwise low doses of general anesthetics, which induce sedation but not anesthesia, to patients to reduce adverse effects of ECT.[39]
While there are no absolute contraindications for ECT, there is increased risk for patients who have unstable or severe cardiovascular conditions or aneurysms; who have recently had a stroke; who have increased intracranial pressure (for instance, due to a solid brain tumor), or who have severe pulmonary conditions, or who are generally at high risk for receiving anesthesia.[9]: 30
In adolescents, ECT is highly efficient for several psychiatric disorders, with few and relatively benign adverse effects.[40][41][42]
Cognitive impairment
Cognitive impairment is sometimes noticed after ECT.[43][44][45][46] It has been claimed by some non-medical authors that retrograde amnesia occurs to some extent in almost all patients receiving ECT.[47] However, most experts consider this adverse effect relatively uncommon.[48] The American Psychiatric Association (APA) report in 2001 acknowledges: "In some patients the recovery from retrograde amnesia will be incomplete, and evidence has shown that ECT can result in persistent or permanent memory loss".[9] After treatment, drug therapy is usually continued and some patients will continue to receive maintenance ECT treatments.[5] It is the purported effects of ECT on long-term memory that give rise to much of the concern surrounding its use.[49] However, the methods used to measure memory loss are generally poor, and their application to people with depression, who have cognitive deficits including problems with memory, have been problematic.[48]
The acute effects of ECT can include amnesia, both retrograde (for events occurring before the treatment) and anterograde (for events occurring after the treatment).[50] Memory loss and confusion are more pronounced with bilateral electrode placement rather than unilateral, and with outdated sine-wave rather than brief-pulse currents. The use of either constant or pulsing electrical impulses also varied the memory loss results in patients. Patients who received pulsing electrical impulses, as opposed to a steady flow, seemed to incur less memory loss. The vast majority of modern treatment uses brief pulse currents.[50]
Retrograde amnesia is most marked for events occurring in the weeks or months before treatment, with one study showing that although some people lose memories from years prior to treatment, recovery of such memories was "virtually complete" by seven months post-treatment, with the only enduring loss being memories in the weeks and months prior to the treatment.[51][52] Anterograde memory loss is usually limited to the time of treatment itself or shortly afterwards. In the weeks and months following ECT these memory problems gradually improve, but some people have persistent losses, especially with bilateral ECT.[1][50] One published review summarizing the results of questionnaires about subjective memory loss found that between 29% and 55% of respondents believed they experienced long-lasting or permanent memory changes.[53] In 2000, American psychiatrist Sarah Lisanby and colleagues found that bilateral ECT left patients with more persistently impaired memory of public events as compared to right unilateral ECT.[49]
Effects on brain structure
Considerable controversy exists over the effects of ECT on brain tissue, although a number of mental health associations—including the APA—have concluded that there is no evidence that ECT causes structural brain damage.[9][20] A 1999 report by the US Surgeon General states: "The fears that ECT causes gross structural brain pathology have not been supported by decades of methodologically sound research in both humans and animals."[54]
Many expert proponents of ECT maintain that the procedure is safe and does not cause brain damage. Dr. Charles Kellner, a prominent ECT researcher and former chief editor of the Journal of ECT, stated in a 2007 interview that, "There are a number of well-designed studies that show ECT does not cause brain damage and numerous reports of patients who have received a large number of treatments over their lifetime and have suffered no significant problems due to ECT."[55] Kellner cites a study purporting to show an absence of cognitive impairment in eight subjects after more than 100 lifetime ECT treatments.[56] Kellner stated "Rather than cause brain damage, there is evidence that ECT may reverse some of the damaging effects of serious psychiatric illness." Two meta-analyses find that ECT is associated with brain matter growth.[57][58]
Effects in pregnancy
If steps are taken to decrease potential risks, ECT is generally accepted to be relatively safe during all trimesters of pregnancy, particularly when compared to pharmacological treatments.[10][59] Suggested preparation for ECT during pregnancy includes a pelvic examination, discontinuation of nonessential anticholinergic medication, uterine tocodynamometry, intravenous hydration, and administration of a nonparticulate antacid. During ECT, elevation of the pregnant woman's right hip, external fetal cardiac monitoring, intubation, and avoidance of excessive hyperventilation are recommended.[10] In many instances of active mood disorder during pregnancy, the risks of untreated symptoms may outweigh the risks of ECT. Potential complications of ECT during pregnancy can be minimized by modifications in technique. The use of ECT during pregnancy requires thorough evaluation of the patient's capacity for informed consent.[60]
Effects on the heart
ECT can cause a lack of blood flow and oxygen to the heart, heart arrhythmia, and "persistent asystole". Deaths, however, are very rare after ECT: 6 per 100,000 treatments. If they do occur, cardiovascular complications are considered as causal in about 30%.[61]
Procedure
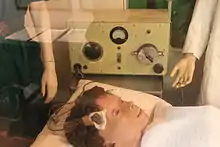

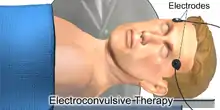
The placement of electrodes, as well as the dose and duration of the stimulation is determined on a per-patient basis.[1]: 1881
In unilateral ECT, both electrodes are placed on the same side of the patient's head. Unilateral ECT may be used first to minimize side effects such as memory loss.
In bilateral ECT, the two electrodes are placed on opposite sides of the head. Usually bitemporal placement is used, whereby the electrodes are placed on the temples. Uncommonly bifrontal placement is used; this involves positioning the electrodes on the patient's forehead, roughly above each eye.
Unilateral ECT is thought to cause fewer cognitive effects than bilateral treatment, but is less effective unless administered at higher doses.[1]: 1881 Most patients in the US[62] and almost all in the UK[63][64][65] receive bilateral ECT.
The electrodes deliver an electrical stimulus. The stimulus levels recommended for ECT are in excess of an individual's seizure threshold: about one and a half times seizure threshold for bilateral ECT and up to 12 times for unilateral ECT.[1]: 1881 Below these levels treatment may not be effective in spite of a seizure, while doses massively above threshold level, especially with bilateral ECT, expose patients to the risk of more severe cognitive impairment without additional therapeutic gains.[66] Seizure threshold is determined by trial and error ("dose titration"). Some psychiatrists use dose titration, some still use "fixed dose" (that is, all patients are given the same dose) and others compromise by roughly estimating a patient's threshold according to age and sex.[62] Older men tend to have higher thresholds than younger women, but it is not a hard and fast rule, and other factors, for example drugs, affect seizure threshold.
Immediately prior to treatment, a patient is given a short-acting anesthetic such as methohexital, etomidate, or thiopental,[1] a muscle relaxant such as suxamethonium (succinylcholine), and occasionally atropine to inhibit salivation.[1]: 1882 In a minority of countries such as Japan,[67] India,[68] and Nigeria,[69] ECT may be used without anesthesia. The Union Health Ministry of India recommended a ban on ECT without anesthesia in India's Mental Health Care Bill of 2010 and the Mental Health Care Bill of 2013.[70][71] The practice was abolished in Turkey's largest psychiatric hospital in 2008.[72]
The patient's EEG, ECG, and blood oxygen levels are monitored during treatment.[1]: 1882
ECT is usually administered three times a week, on alternate days, over a course of two to four weeks.[1]: 1882–1883
Neuroimaging prior to ECT
Neuroimaging prior to ECT may be useful for detecting intracranial pressure or mass given that patients respond less when one of these conditions exist. Nonetheless, it is not indicated due to high cost and low prevalence of these conditions in patients needing ECT.[73]
Concurrent pharmacotherapy
Whether psychiatric medications are terminated prior to treatment or maintained, varies.[1]: 1885 [74] However, drugs that are known to cause toxicity in combination with ECT, such as lithium, are discontinued, and benzodiazepines, which increase the seizure threshold,[75] are either discontinued, a benzodiazepine antagonist is administered at each ECT session, or the ECT treatment is adjusted accordingly.[1]: 1875, 1879
A 2009 RCT provides some evidence indicating that concurrent use of some antidepressant improves ECT efficacy.[17]
Course
ECT is usually done from 6 to 12 times in 2 to 4 weeks but can sometimes exceed 12 rounds.[17] It is also recommended to not do ECT more than 3 times per week.[17]
Treatment team
In the US, the medical team performing the procedure typically consists of a psychiatrist, an anesthetist, an ECT treatment nurse or qualified assistant, and one or more recovery nurses.[9]: 109 Medical trainees may assist, but only under the direct supervision of credentialed attending physicians and staff.[9]: 110
Devices
.jpg.webp)
Most modern ECT devices deliver a brief-pulse current, which is thought to cause fewer cognitive effects than the sine-wave currents which were originally used in ECT.[1] A small minority of psychiatrists in the US still use sine-wave stimuli.[62] Sine-wave is no longer used in the UK or Ireland.[65] Typically, the electrical stimulus used in ECT is about 800 milliamps and has up to several hundred watts, and the current flows for between one and six seconds.[66]
In the US, ECT devices are manufactured by two companies, Somatics, which is owned by psychiatrists Richard Abrams and Conrad Swartz, and Mecta.[76] In the UK, the market for ECT devices was long monopolized by Ectron Ltd, which was set up by psychiatrist Robert Russell.[77]
Mechanism of action
Despite decades of research, the exact mechanism of action of ECT remains elusive. Neuroimaging studies in people who have had ECT, investigating differences between responders and nonresponders, and people who relapse, find that responders have anticonvulsant effects mostly in the frontal lobes, which corresponds to immediate responses, and neurotrophic effects primarily in the medial temporal lobe. The anticonvulsant effects are decreased blood flow and decreased metabolism, while the neurotrophic effects are opposite – increased perfusion and metabolism, as well as increased volume of the hippocampus.[13]
Another proposed mechanism of action is that the seizures induced by ECT cause a profound change in sleep architecture (a reversible inhibition of REM sleep); it is this change in the state of the organism that drives the therapeutic effects of ECT and not any simple change in the release of neurotransmitters, neurotrophic factors and/or hormones.[78]
Use
As of 2001, it was estimated that about one million people received ECT annually.[79]
There is wide variation in ECT use between different countries, different hospitals, and different psychiatrists.[1][79] International practice varies considerably from widespread use of the therapy in many Western countries to a small minority of countries that do not use ECT at all, such as Slovenia.[80]
About 70 percent of ECT patients are women.[1] This may be because women are more likely to be diagnosed with depression.[1][81] Older and more affluent patients are also more likely to receive ECT. The use of ECT is not as common in ethnic minorities.[81][82]
Sarah Hall reports, "ECT has been dogged by conflict between psychiatrists who swear by it, and some patients and families of patients who say that their lives have been ruined by it. It is controversial in some European countries such as the Netherlands and Italy, where its use is severely restricted".[83]
United States
ECT became popular in the US in the 1940s. At the time, psychiatric hospitals were overrun with patients whom doctors were desperate to treat and cure. Whereas lobotomies would reduce a patient to a more manageable submissive state, ECT helped to improve mood in those with severe depression. A survey of psychiatric practice in the late 1980s found that an estimated 100,000 people received ECT annually, with wide variation between metropolitan statistical areas.[84] Accurate statistics about the frequency, context and circumstances of ECT in the US are difficult to obtain because only a few states have reporting laws that require the treating facility to supply state authorities with this information.[85] In 13 of the 50 states, the practice of ECT is regulated by law.[86] In the mid-1990s in Texas, ECT was used in about one third of psychiatric facilities and given to about 1,650 people annually.[81] Usage of ECT has since declined slightly; in 2000–01 ECT was given to about 1,500 people aged from 16 to 97 (in Texas it is illegal to give ECT to anyone under sixteen).[87] ECT is more commonly used in private psychiatric hospitals than in public hospitals, and minority patients are underrepresented in the ECT statistics.[1] In the United States, ECT is usually given three times a week; in the United Kingdom, it is usually given twice a week.[1] Occasionally it is given on a daily basis.[1] A course usually consists of 6–12 treatments, but may be more or fewer. Following a course of ECT some patients may be given continuation or maintenance ECT with further treatments at weekly, fortnightly or monthly intervals.[1] A few psychiatrists in the US use multiple-monitored ECT (MMECT), where patients receive more than one treatment per anesthetic.[1] Electroconvulsive therapy is not a required subject in US medical schools and not a required skill in psychiatric residency training. Privileging for ECT practice at institutions is a local option: no national certification standards are established, and no ECT-specific continuing training experiences are required of ECT practitioners.[88]
United Kingdom
In the UK in 1980, an estimated 50,000 people received ECT annually, with use declining steadily since then[89] to about 12,000 per annum in 2002.[90] It is still used in nearly all psychiatric hospitals, with a survey of ECT use from 2002 finding that 71 percent of patients were women and 46 percent were over 65 years of age. Eighty-one percent had a diagnosis of mood disorder; schizophrenia was the next most common diagnosis. Sixteen percent were treated without their consent.[90] In 2003, the National Institute for Health and Care Excellence, a government body which was set up to standardize treatment throughout the National Health Service in England and Wales, issued guidance on the use of ECT. Its use was recommended "only to achieve rapid and short-term improvement of severe symptoms after an adequate trial of treatment options has proven ineffective and/or when the condition is considered to be potentially life-threatening in individuals with severe depressive illness, catatonia or a prolonged manic episode".[91]
The guidance received a mixed reception. It was welcomed by an editorial in the British Medical Journal[92] but the Royal College of Psychiatrists launched an unsuccessful appeal.[93] The NICE guidance, as the British Medical Journal editorial points out, is only a policy statement and psychiatrists may deviate from it if they see fit. Adherence to standards has not been universal in the past. A survey of ECT use in 1980 found that more than half of ECT clinics failed to meet minimum standards set by the Royal College of Psychiatrists, with a later survey in 1998 finding that minimum standards were largely adhered to, but that two-thirds of clinics still fell short of current guidelines, particularly in the training and supervision of junior doctors involved in the procedure.[94] A voluntary accreditation scheme, ECTAS, was set up in 2004 by the Royal College, and as of 2017 the vast majority of ECT clinics in England, Wales, Northern Ireland and the Republic of Ireland have signed up.[95]
The Mental Health Act 2007 allows people to be treated against their will. This law has extra protections regarding ECT. A patient capable of making the decision can decline the treatment, and in that case treatment cannot be given unless it will save that patient's life or is immediately necessary to prevent deterioration of the patient's condition. A patient may not be capable of making the decision (they "lack capacity"), and in that situation ECT can be given if it is appropriate and also if there are no advance directives that prevent the use of ECT.[96]
China
ECT was introduced in China in the early 1950s and while it was originally practiced without anesthesia, as of 2012 almost all procedures were conducted with it. As of 2012, there are approximately 400 ECT machines in China, and 150,000 ECT treatments are performed each year.[97] Chinese national practice guidelines recommend ECT for the treatment of schizophrenia, depressive disorders, and bipolar disorder and in the Chinese literature, ECT is an effective treatment for schizophrenia and mood disorders.[97]
Although the Chinese government stopped classifying homosexuality as an illness in 2001, electroconvulsive therapy is still used by some establishments as a form of "conversion therapy".[98][99] Alleged Internet addiction (or general unruliness) in adolescents is also known to have been treated with ECT, sometimes without anestheia, most notably by Yang Yongxin. The practice was banned in 2009 after news on Yang broke out.[100]
History

As early as the 16th century, agents to induce seizures were used to treat psychiatric conditions. In 1785, the therapeutic use of seizure induction was documented in the London Medical and Surgical Journal.[1][101] As to its earliest antecedents one doctor claims 1744 as the dawn of electricity's therapeutic use, as documented in the first issue of Electricity and Medicine. Treatment and cure of hysterical blindness was documented eleven years later. Benjamin Franklin wrote that an electrostatic machine cured "a woman of hysterical fits." By 1801, James Lind[102] as well as Giovanni Aldini had used galvanism to treat patients with various mental disorders.[103] G.B.C. Duchenne, the mid-19th century "Father of Electrotherapy", said its use was integral to a neurological practice.[104]
In the second half of the 19th century, such efforts were frequent enough in British asylums as to make it notable.[105]
Convulsive therapy was introduced in 1934 by Hungarian neuropsychiatrist Ladislas J. Meduna who, believing mistakenly that schizophrenia and epilepsy were antagonistic disorders, induced seizures first with camphor and then metrazol (cardiazol).[106][107] Meduna is thought to be the father of convulsive therapy.[108] In 1937, the first international meeting on schizophrenia and convulsive therapy was held in Switzerland by the Swiss psychiatrist Max Müller.[109] The proceedings were published in the American Journal of Psychiatry and, within three years, cardiazol convulsive therapy was being used worldwide.[107] Italian professor of neuropsychiatry Ugo Cerletti, who had been using electric shocks to produce seizures in animal experiments, and his assistant Lucio Bini at Sapienza University of Rome developed the idea of using electricity as a substitute for metrazol in convulsive therapy and, in 1938, experimented for the first time on a person affected by delusions. It was believed early on that inducing convulsions aided in helping those with severe schizophrenia but later found to be most useful with affective disorders such as depression. Cerletti had noted a shock to the head produced convulsions in dogs. The idea to use electroshock on humans came to Cerletti when he saw how pigs were given an electric shock before being butchered to put them in an anesthetized state.[110] Cerletti and Bini practiced until they felt they had the right parameters needed to have a successful human trial. Once they started trials on patients, they found that after 10-20 treatments the results were significant. Patients had much improved. A positive side effect to the treatment was retrograde amnesia. It was because of this side effect that patients could not remember the treatments and had no ill feelings toward it.[110] ECT soon replaced metrazol therapy all over the world because it was cheaper, less frightening and more convenient.[111] Cerletti and Bini were nominated for a Nobel Prize but did not receive one. By 1940, the procedure was introduced to both England and the US. In Germany and Austria, it was promoted by Friedrich Meggendorfer. Through the 1940s and 1950s, the use of ECT became widespread. At the time the ECT device was patented and commercialized abroad, the two Italian inventors had competitive tensions that damaged their relationship.[112] In the 1960s, despite a climate of condemnation, the original Cerletti-Bini ECT apparatus prototype was hotly contended by scientific museums between Italy and the USA.[113] The ECT apparatus prototype is now owned and displayed by the Sapienza Museum of the History of Medicine in Rome.[113]
In the early 1940s, in an attempt to reduce the memory disturbance and confusion associated with treatment, two modifications were introduced: the use of unilateral electrode placement and the replacement of sinusoidal current with brief pulse. It took many years for brief-pulse equipment to be widely adopted.[114] In the 1940s and early 1950s ECT, was usually given in "unmodified" form, without muscle relaxants, and the seizure resulted in a full-scale convulsion. A rare but serious complication of unmodified ECT was fracture or dislocation of the long bones. In the 1940s, psychiatrists began to experiment with curare, the muscle-paralysing South American poison, in order to modify the convulsions. The introduction of suxamethonium (succinylcholine), a safer synthetic alternative to curare, in 1951 led to the more widespread use of "modified" ECT. A short-acting anesthetic was usually given in addition to the muscle relaxant in order to spare patients the terrifying feeling of suffocation that can be experienced with muscle relaxants.[114]
The steady growth of antidepressant use along with negative depictions of ECT in the mass media led to a marked decline in the use of ECT during the 1950s to the 1970s. The Surgeon General stated there were problems with electroshock therapy in the initial years before anesthesia was routinely given, and that "these now-antiquated practices contributed to the negative portrayal of ECT in the popular media."[115] The New York Times described the public's negative perception of ECT as being caused mainly by one movie: "For Big Nurse in One Flew Over the Cuckoo's Nest, it was a tool of terror, and, in the public mind, shock therapy has retained the tarnished image given it by Ken Kesey's novel: dangerous, inhumane and overused".[116]
In 1976, Dr. Blatchley demonstrated the effectiveness of his constant current, brief pulse device ECT. This device eventually largely replaced earlier devices because of the reduction in cognitive side effects, although as of 2012 some ECT clinics still were using sine-wave devices.[79] The 1970s saw the publication of the first American Psychiatric Association (APA) task force report on electroconvulsive therapy (to be followed by further reports in 1990 and 2001). The report endorsed the use of ECT in the treatment of depression. The decade also saw criticism of ECT.[117] Specifically, critics pointed to shortcomings such as noted side effects, the procedure being used as a form of abuse, and uneven application of ECT. The use of ECT declined until the 1980s, "when use began to increase amid growing awareness of its benefits and cost-effectiveness for treating severe depression".[115] In 1985, the National Institute of Mental Health and National Institutes of Health convened a consensus development conference on ECT and concluded that, while ECT was the most controversial treatment in psychiatry and had significant side-effects, it had been shown to be effective for a narrow range of severe psychiatric disorders.[118]
Because of the backlash noted previously, national institutions reviewed past practices and set new standards. In 1978, the American Psychiatric Association released its first task force report in which new standards for consent were introduced and the use of unilateral electrode placement was recommended. The 1985 NIMH Consensus Conference confirmed the therapeutic role of ECT in certain circumstances. The American Psychiatric Association released its second task force report in 1990 where specific details on the delivery, education, and training of ECT were documented. Finally, in 2001 the American Psychiatric Association released its latest task force report.[9] This report emphasizes the importance of informed consent, and the expanded role that the procedure has in modern medicine. By 2017, ECT was routinely covered by insurance companies for providing the "biggest bang for the buck" for otherwise intractable cases of severe mental illness, was receiving favorable media coverage, and was being provided in regional medical centers.[119]
Though ECT use declined with the advent of modern antidepressants, there has been a resurgence of ECT with new modern technologies and techniques.[120] Modern shock voltage is given for a shorter duration of 0.5 milliseconds where conventional brief pulse is 1.5 milliseconds.[121]
Society and culture
Controversy
Surveys of public opinion, the testimony of former patients, legal restrictions on the use of ECT and disputes as to the efficacy, ethics and adverse effects of ECT within the psychiatric and wider medical community indicate that the use of ECT remains controversial.[122][123][124][125][126][127][128] This is reflected in the January 2011 vote by the FDA's Neurological Devices Advisory Panel to recommend that FDA maintain ECT devices in the Class III device category for high risk devices, except for patients with catatonia, major depressive disorder, and bipolar disorder.[7] This may result in the manufacturers of such devices having to do controlled trials on their safety and efficacy for the first time.[5][129][130] In justifying their position, panelists referred to the memory loss associated with ECT and the lack of long-term data.[131]
Informed consent
The World Health Organization (2005) advises that ECT should be used only with the informed consent of the patient (or their guardian if their incapacity to consent has been established).[14]
In the US, this doctrine places a legal obligation on a doctor to make a patient aware of the reason for treatment, the risks and benefits of a proposed treatment, the risks and benefits of alternative treatment, and the risks and benefits of receiving no treatment. The patient is then given the opportunity to accept or reject the treatment. The form states how many treatments are recommended and also makes the patient aware that consent may be revoked and treatment discontinued at any time during a course of ECT.[8] The US Surgeon General's Report on Mental Health states that patients should be warned that the benefits of ECT are short-lived without active continuation treatment in the form of drugs or further ECT, and that there may be some risk of permanent, severe memory loss after ECT.[8] The report advises psychiatrists to involve patients in discussion, possibly with the aid of leaflets or videos, both before and during a course of ECT.
According to the US Surgeon General, involuntary treatment is uncommon in the US and is typically used only in cases of great extremity, and only when all other treatment options have been exhausted. The use of ECT is believed to be a potentially life-saving treatment.[54]
In one of the few jurisdictions where recent statistics on ECT usage are available, a national audit of ECT by the Scottish ECT Accreditation Network indicated that 77% of patients who received the treatment in 2008 were capable of giving informed consent.[132]
In the UK, in order for consent to be valid it requires an explanation in "broad terms" of the nature of the procedure and its likely effects.[133] One review from 2005 found that only about half of patients felt they were given sufficient information about ECT and its adverse effects[134] and another survey found that about fifty percent of psychiatrists and nurses agreed with them.[135]
A 2005 study published in the British Journal of Psychiatry described patients' perspectives on the adequacy of informed consent before ECT.[134] The study found that "About half (45–55%) of patients reported they were given an adequate explanation of ECT, implying a similar percentage felt they were not." The authors also stated:
Approximately a third did not feel they had freely consented to ECT even when they had signed a consent form. The proportion who feel they did not freely choose the treatment has actually increased over time. The same themes arise whether the patient had received treatment a year ago or 30 years ago. Neither current nor proposed safeguards for patients are sufficient to ensure informed consent with respect to ECT, at least in England and Wales.[134]
Involuntary ECT
Procedures for involuntary ECT vary from country to country depending on local mental health laws.
United States
In most states in the US, a judicial order following a formal hearing is needed before a patient can be forced to undergo involuntary ECT.[8] However, ECT can also be involuntarily administered in situations with less immediate danger. Suicidal intent is a common justification for its involuntary use, especially when other treatments are ineffective.[8]
United Kingdom
Until 2007 in England and Wales, the Mental Health Act 1983 allowed the use of ECT on detained patients whether or not they had capacity to consent to it. However, following amendments which took effect in 2007, ECT may not generally be given to a patient who has capacity and refuses it, irrespective of his or her detention under the Act.[136] In fact, even if a patient is deemed to lack capacity, if they made a valid advance decision refusing ECT then they should not be given it; and even if they do not have an advance decision, the psychiatrist must obtain an independent second opinion (which is also the case if the patient is under age of consent).[137] However, there is an exception regardless of consent and capacity; under Section 62 of the Act, if the treating psychiatrist says the need for treatment is urgent they may start a course of ECT without authorization.[138] From 2003 to 2005, about 2,000 people a year in England and Wales were treated without their consent under the Mental Health Act.[139] Concerns have been raised by the official regulator that psychiatrists are too readily assuming that patients have the capacity to consent to their treatments, and that there is a worrying lack of independent advocacy.[140] In Scotland, the Mental Health (Care and Treatment) (Scotland) Act 2003 also gives patients with capacity the right to refuse ECT.[141]
Regulation
In the US, ECT devices came into existence prior to medical devices being regulated by the Food and Drug Administration. In 1976, the Medical Device Regulation Act required the FDA to retrospectively review already existing devices, classify them, and determine whether clinical trials were needed to prove efficacy and safety. The FDA initially classified the devices used to administer ECT as Class III medical devices. In 2014, the American Psychiatric Association petitioned the FDA to reclassify ECT devices from Class III (high-risk) to Class II (medium-risk), which would significantly improve access to an effective and potentially lifesaving treatment. A similar reclassification proposal in 2010 met significant resistance from anti-psychiatry groups and did not pass.[142] In 2018, the FDA re-classified ECT devices as Class II devices when used to treat catatonia or a severe major depressive episode associated with major depressive disorder or bipolar disorder.[7]
Public perception
A questionnaire survey of 379 members of the general public in Australia indicated that more than 60% of respondents had some knowledge about the main aspects of ECT. Participants were generally opposed to the use of ECT on depressed individuals with psychosocial issues, on children, and on involuntary patients. Public perceptions of ECT were found to be mainly negative.[128] A sample of the general public, medical students, and psychiatry trainees in the United Kingdom found that the psychiatry trainees were more knowledgeable and had more favorable opinions of ECT than did the other groups.[143] More members of the general public believed that ECT was used for control or punishment purposes than medical students or psychiatry trainees.[143]
Famous cases
Ernest Hemingway, an American author, died by suicide shortly after ECT at the Mayo Clinic in 1961.[144] He is reported to have said to his biographer, "Well, what is the sense of ruining my head and erasing my memory, which is my capital, and putting me out of business? It was a brilliant cure but we lost the patient."[145]
Robert Pirsig had a nervous breakdown and spent time in and out of psychiatric hospitals between 1961 and 1963.[146] He was diagnosed with paranoid schizophrenia and clinical depression as a result of an evaluation conducted by psychoanalysts, and was treated with electroconvulsive therapy on numerous occasions,[147] a treatment he discusses in his novel, Zen and the Art of Motorcycle Maintenance.[148]
Thomas Eagleton, United States Senator from Missouri, was dropped from the Democratic ticket in the 1972 United States Presidential Election as the party's vice presidential candidate after it was revealed that he had received electroshock treatment in the past for depression. Presidential nominee George McGovern replaced him with Sargent Shriver, and later went on to lose by a landslide to Richard Nixon.
American surgeon and award-winning author Sherwin B. Nuland is another notable person who has undergone ECT.[149] In his 40s, this successful surgeon's depression became so severe that he had to be institutionalized. After exhausting all treatment options, a young resident assigned to his case suggested ECT, which ended up being successful.[150]
Author David Foster Wallace also received ECT for many years, beginning as a teenager, before his suicide at age 46.[151]
New Zealand author Janet Frame experienced both insulin coma therapy and ECT (but without the use of anesthesia or muscle relaxants).[152] She wrote about this in her autobiography, An Angel at My Table (1984),[152] which was later adapted into a film (1990).[153]
American actor Carrie Fisher wrote about her experience with memory loss after ECT treatments in her memoir Wishful Drinking.[154]
Fictional examples
Electroconvulsive therapy has been depicted in fiction, including fictional works partly based on true experiences. These include Sylvia Plath's autobiographical novel, The Bell Jar, Ken Loach's film Family Life, and Ken Kesey's novel One Flew Over the Cuckoo's Nest; Kesey's novel is a direct product of his time working the graveyard shift as an orderly at a mental health facility in Menlo Park, California.[155][156]
Special populations
Sex difference
Throughout the history of ECT, women have received it two to three times as often as men.[157] Currently, about 70 percent of ECT patients are women.[1] This may be because women are more likely to be diagnosed with depression.[1][81] A 1974 study of ECT in Massachusetts reported that women made up 69 percent of those given ECT.[158] The Ministry of Health in Canada reported that from 1999 until 2000 in the province of Ontario, women were 71 percent of those given ECT in provincial psychiatric institutions, and 75 percent of the total ECT given was given to women.[159]
References
- Rudorfer, MV, Henry, ME, Sackeim, HA (2003). "Electroconvulsive therapy" Archived 2007-08-10 at the Wayback Machine. In A Tasman, J Kay, JA Lieberman (eds) Psychiatry, Second Edition. Chichester: John Wiley & Sons Ltd, 1865–1901.
- Solano, Juanjo (2009-04-20). "Electroconvulsive Therapy" (PDF). p. 4. Retrieved 2022-05-17.
{{cite web}}: CS1 maint: url-status (link) - Rudorfer, M. V., Henry, M. E., & Sackheim, H. A. (1997). "Electroconvulsive therapy". In A. Tasman, J. & J. A Lieberman (Eds.), Psychiatry (1535–1556)
- Beloucif S (2013). "Informed consent for special procedures: electroconvulsive therapy and psychosurgery". Curr Opin Anesthesiol. 26 (2): 182–185. doi:10.1097/ACO.0b013e32835e7380. PMID 23385317. S2CID 36643014.
- FDA. FDA Executive Summary. Prepared for the January 27–28, 2011 meeting of the Neurological Devices Panel Meeting to Discuss the Classification of Electroconvulsive Therapy Devices (ECT). Quote, p. 38: "Three major practice guidelines have been published on ECT. These guidelines include: APA Task Force on ECT (2001); Third report of the Royal College of Psychiatrists' Special Committee on ECT (2004); National Institute for Health and Clinical Excellence (NICE 2003; NICE 2009). There is significant agreement between the three sets of recommendations."
- "CFR – Code of Federal Regulations Title 21".
- US Food and Drug Administration (2018-12-21). "FDA In Brief: FDA takes action to ensure regulation of electroconvulsive therapy devices better protects patients, reflects current understanding of safety and effectiveness" (Press release).
- Surgeon General (1999). Mental Health: A Report of the Surgeon General, chapter 4.
- American Psychiatric Association; Committee on Electroconvulsive Therapy; Richard D. Weiner (chairperson); et al. (2001). The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging (2nd ed.). Washington, DC: American Psychiatric Publishing. ISBN 978-0890422069.
- Pompili M, et al. (Dec 2014). "Electroconvulsive treatment during pregnancy: a systematic review". Expert Rev Neurother. 14 (12): 1377–1390. doi:10.1586/14737175.2014.972373. PMID 25346216. S2CID 31209001.
- Margarita Tartakovsky (2012) Psych Central. 5 Outdated Beliefs About ECT Archived 2013-08-08 at the Wayback Machine
- Kolshus E, Jelovac A, McLoughlin DM (2017). "Bitemporal v. high-dose right unilateral electroconvulsive therapy for depression: a systematic review and meta-analysis of randomized controlled trials" (PDF). Psychol Med. 47 (3): 518–530. doi:10.1017/S0033291716002737. PMID 27780482. S2CID 10711085.
- Abbott CC, et al. (Mar 2014). "A review of longitudinal electroconvulsive therapy: neuroimaging investigations". J Geriatr Psychiatry Neurol. 27 (1): 33–46. doi:10.1177/0891988713516542. PMC 6624835. PMID 24381234.
- World Health Organisation (2005). WHO Resource Book on Mental Health, Human Rights and Legislation Archived 2006-12-06 at the Wayback Machine. Geneva, 64.
- Benevides, Teal W; Shore, Stephen M; Andresen, May-Lynn; Caplan, Reid; Cook, Barb; Gassner, Dena L; Erves, Jasmine M; Hazlewood, Taylor M; King, M Caroline; Morgan, Lisa; Murphy, Lauren E (2020). "Interventions to address health outcomes among autistic adults: A systematic review". Autism. 24 (6): 1345–1359. doi:10.1177/1362361320913664. ISSN 1362-3613. PMC 7787674. PMID 32390461. S2CID 218586379.
- Lipsman N, et al. (Jan 2014). "Neuromodulation for treatment-refractory major depressive disorder". Canadian Medical Association Journal. 186 (1): 33–39. doi:10.1503/cmaj.121317. PMC 3883821. PMID 23897945.
- Tasman, Allan; Kay, Jerald; Lieberman, Jeffrey A.; First, Michael B.; Riba, Michelle B., eds. (2015). Psychiatry. Chichester, UK: John Wiley & Sons, Ltd. doi:10.1002/9781118753378. ISBN 978-1118753378.
- Bolwig, Tom G (2005). "First-line Use of ECT". The Journal of ECT. Ovid Technologies (Wolters Kluwer Health). 21 (1): 51. doi:10.1097/01.yct.0000158271.45828.76. ISSN 1095-0680. PMID 15791182.
- Fitzgerald, P. B (2013). "Non-pharmacological biological treatment approaches to difficult-to-treat depression". The Medical Journal of Australia. 199 (6 Suppl): S48–51. doi:10.5694/mja12.10509. PMID 25370288. S2CID 204073048.
- "Depression in adults: The treatment and management of depression in adults. NICE guidelines CG90". National Institute for Clinical Excellence. 2009.
- Murray ED, Buttner N, Price BH (2012). "Depression and Psychosis in Neurological Practice". In Bradley WG, Daroff RB, Fenichel GM, Jankovic J (eds.). Bradley's Neurology in Clinical Practice: Expert Consult. Vol. 1 (6th ed.). Philadelphia: Elsevier/Saunders. pp. 114–115. ISBN 978-1437704341.
- Dierckx, Bram; Heijnen, Willemijn T; van den Broek, Walter W; Birkenhäger, Tom K (2012). "Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis". Bipolar Disorders. Wiley. 14 (2): 146–150. doi:10.1111/j.1399-5618.2012.00997.x. ISSN 1398-5647. PMID 22420590. S2CID 44280002.
- Pagnin D, de Queiroz V, Pini S, Cassano GB (2004). "Efficacy of ECT in depression: a meta-analytic review". The Journal of ECT. 20 (1): 13–20. doi:10.1097/00124509-200403000-00004. PMID 15087991. S2CID 25843283.
- UK ECT Review Group (2003). "Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis". The Lancet. 361 (9360): 799–808. doi:10.1016/S0140-6736(03)12705-5. PMID 12642045. S2CID 28964580.
- Micallef-Trigona B (2014). "Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: a systematic review and meta-analysis". Depress Res Treat. 2014: 135049. doi:10.1155/2014/135049. PMC 4131106. PMID 25143831.
- Shelton, Richard C.; Osuntokun, Olawale; Heinloth, Alexandra N.; Corya, Sara A. (2010). "Therapeutic Options for Treatment-Resistant Depression". CNS Drugs. Springer Science and Business Media LLC. 24 (2): 131–161. doi:10.2165/11530280-000000000-00000. ISSN 1172-7047. PMID 20088620. S2CID 32936223.
- Jelovac A, et al. (Nov 2013). "Relapse following successful electroconvulsive therapy for major depression: a meta-analysis". Neuropsychopharmacology. 38 (12): 2467–2474. doi:10.1038/npp.2013.149. PMC 3799066. PMID 23774532.
- Lambrichts, S; Detraux, J; Vansteelandt, K; Nordenskjöld, A; Obbels, J; Schrijvers, D; Sienaert, P (2021). "Does lithium prevent relapse following successful electroconvulsive therapy for major depression? A systematic review and meta-analysis". Acta Psychiatrica Scandinavica. 143 (4): 294–306. doi:10.1111/acps.13277. ISSN 0001-690X. PMID 33506961. S2CID 231759831.
- Sienaert P, et al. (Dec 2014). "A clinical review of the treatment of catatonia". Front Psychiatry. 5: 181. doi:10.3389/fpsyt.2014.00181. PMC 4260674. PMID 25538636.
- Leroy, Arnaud; Naudet, Florian; Vaiva, Guillaume; Francis, Andrew; Thomas, Pierre; Amad, Ali (2018). "Is electroconvulsive therapy an evidence-based treatment for catatonia? A systematic review and meta-analysis". European Archives of Psychiatry and Clinical Neuroscience. 268 (7): 675–687. doi:10.1007/s00406-017-0819-5. ISSN 0940-1334. PMID 28639007. S2CID 4013882.
- DeJong H, et al. (Sep 2014). "A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders". J Autism Dev Disord. 44 (9): 2127–2136. doi:10.1007/s10803-014-2085-y. PMID 24643578. S2CID 22002956.
- NICE Guidance on the use of electroconvulsive therapy. NICE technology appraisals TA59. Published date: April 2003
- Kanba S, Kato T, Terao T, Yamada K (Jul 2013). "Guideline for treatment of bipolar disorder by the Japanese Society of Mood Disorders, 2012". Psychiatry Clin Neurosci. 67 (5): 285–300. doi:10.1111/pcn.12060. PMID 23773266. S2CID 2058163.
- Malhi GS, et al. (Dec 2012). "Mania: diagnosis and treatment recommendations". Curr Psychiatry Rep. 14 (6): 676–686. doi:10.1007/s11920-012-0324-5. PMID 22986995. S2CID 37771648.
- Tharyan P, Adams CE (2005). Tharyan P (ed.). "Electroconvulsive therapy for schizophrenia". The Cochrane Database of Systematic Reviews (2): CD000076. doi:10.1002/14651858.CD000076.pub2. PMID 15846598.
- Penders TM, Gestring RE, Vilensky DA (November 2012). "Intoxication delirium following use of synthetic cathinone derivatives". The American Journal of Drug and Alcohol Abuse. 38 (6): 616–617. doi:10.3109/00952990.2012.694535. PMID 22783894. S2CID 207428569.
- Penders TM, et al. (December 2013). "Electroconvulsive Therapy Improves Persistent Psychosis After Repeated Use of Methylenedioxypyrovalerone ("Bath Salts")". The Journal of ECT. 29 (4): 59–60. doi:10.1097/YCT.0b013e3182887bc2. PMID 23609518. S2CID 45842375.
- Tørring, N.; Sanghani, S. N.; Petrides, G.; Kellner, C. H.; Østergaard, S. D. (2017). "The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis". Acta Psychiatrica Scandinavica. 135 (5): 388–397. doi:10.1111/acps.12721. ISSN 1600-0447. PMID 28332236. S2CID 31879446.
- Gallegos J; Vaidya P; D'Agati D; et al. (June 2012). "Decreasing adverse outcomes of unmodified electroconvulsive therapy: suggestions and possibilities". J ECT. 28 (2): 77–81. doi:10.1097/YCT.0b013e3182359314. PMID 22531198. S2CID 6423840.
- Neera Ghaziuddin, Garry Walter (eds.): Electroconvulsive Therapy in Children and Adolescents, Oxford University Press, 2013, ISBN 978-0199937899, pp. 161–280.
- Lima, Nádia NR; Nascimento, Vânia B; Peixoto, Jorge AC; Moreira, Marcial M; Neto, Modesto LR; Almeida, José C; Vasconcelos, Carlos AC; Teixeira, Saulo A; Júnior, Jucier G; Junior, Francisco TC; Guimarães, Diego DM; Brasil, Aline Q; Cartaxo, Jesus S; Akerman, Marco; Reis, Alberto OA (2013). "Electroconvulsive therapy use in adolescents: a systematic review". Annals of General Psychiatry. 12 (1): 17. doi:10.1186/1744-859X-12-17. ISSN 1744-859X. PMC 3680000. PMID 23718899.
- Benson NM, Seiner SJ (2019). "Electroconvulsive Therapy in Children and Adolescents: Clinical Indications and Special Considerations". Harv Rev Psychiatry. 27 (6): 354–358. doi:10.1097/HRP.0000000000000236. PMID 31714466. S2CID 207934946.
- Holtzheimer PE 3rd, Mayberg HS (2010). "Deep brain stimulation for treatment-resistant depression". Am J Psychiatry. 167 (12): 1437–1444. doi:10.1176/appi.ajp.2010.10010141. PMC 4413473. PMID 21131410.
- McClintock SM, Choi J, Deng ZD, Appelbaum LG, Krystal AD, Lisanby SH (2014). "Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy". J ECT. 30 (2): 165–176. doi:10.1097/YCT.0000000000000137. hdl:10161/10644. PMC 4143898. PMID 24820942.
- Loo CK, Katalinic N, Smith DJ, Ingram A, Dowling N, Martin D, Addison K, Hadzi-Pavlovic D, Simpson B, Schweitzer I (2014). "A randomized controlled trial of brief and ultrabrief pulse right unilateral electroconvulsive therapy". Int. J. Neuropsychopharmacol. 18 (1): pyu045. doi:10.1093/ijnp/pyu045. PMC 4368876. PMID 25522389.
- Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, McClintock SM, Tobias KG, Martino C, Mueller M, Bailine SH, Fink M, Petrides G (2010). "Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial". Br J Psychiatry. 196 (3): 226–234. doi:10.1192/bjp.bp.109.066183. PMC 2830057. PMID 20194546.
- Read, J; Bentall, R (Oct–Dec 2010). "The effectiveness of electroconvulsive therapy: a literature review" (PDF). Epidemiologia e Psichiatria Sociale. 19 (4): 333–347. doi:10.1017/S1121189X00000671. PMID 21322506. S2CID 15118442. Archived from the original (PDF) on 2012-05-24. Retrieved 2012-01-23.
- Semkovska M, McLoughlin DM (Jun 2013). "Measuring retrograde autobiographical amnesia following electroconvulsive therapy: historical perspective and current issues". J ECT. 29 (2): 127–133. doi:10.1097/YCT.0b013e318279c2c9. PMID 23303426. S2CID 45019739.
- Lisanby SH, Maddox JH, Prudic J, Devanand DP, Sackeim HA (June 2000). "The effects of electroconvulsive therapy on memory of autobiographical and public events". Arch. Gen. Psychiatry. 57 (6): 581–590. doi:10.1001/archpsyc.57.6.581. PMID 10839336.
- Benbow, SM (2004) "Adverse effects of ECT". In AIF Scott (ed.) The ECT Handbook, second edition. Archived 2012-04-21 at the Wayback Machine London: The Royal College of Psychiatrists, pp. 170–174.
- Squire LR, Slater PC, Miller PL (January 1981). "Retrograde amnesia and bilateral electroconvulsive therapy. Long-term follow-up". Arch. Gen. Psychiatry. 38 (1): 89–95. doi:10.1001/archpsyc.1981.01780260091010. PMID 7458573.
- Squire LR, Slater PC (January 1983). "Electroconvulsive therapy and complaints of memory dysfunction: a prospective three-year follow-up study". Br J Psychiatry. 142: 1–8. doi:10.1192/bjp.142.1.1. PMID 6831121. S2CID 22600323.
- Rose, D; Fleischmann, P; Wykes, T; Leese, M; Bindman, J (2003). "Patients' perspectives on electroconvulsive therapy: systematic review". British Medical Journal. 326 (7403): 1363–1365. doi:10.1136/bmj.326.7403.1363. PMC 162130. PMID 12816822.
- "Chapter 4". Mental Health: A Report of the Surgeon General (Report). Office of the Surgeon General (US). Retrieved 2007-12-29.
- Sussman, Norman (March 2007). "In Session with Charles H. Kellner, MD: Current Developments in Electroconvulsive Therapy". Primary Psychiatry. 14 (3): 34–37. Archived from the original on 2011-05-16. Retrieved 2009-10-17.
- Devanand DP; Sackeim HA; et al. (July 1991). "Absence of cognitive impairment after more than 100 lifetime ECT treatments". The American Journal of Psychiatry. 148 (7): 929–932. doi:10.1176/ajp.148.7.929. PMID 2053635.
- Gbyl, K.; Videbech, P. (September 2018). "Electroconvulsive therapy increases brain volume in major depression: a systematic review and meta-analysis". Acta Psychiatrica Scandinavica. 138 (3): 180–195. doi:10.1111/acps.12884. ISSN 1600-0447. PMID 29707778. S2CID 14042369.
- Wilkinson, Samuel T.; Sanacora, Gerard; Bloch, Michael H. (May 2017). "Hippocampal volume changes following electroconvulsive therapy: a systematic review and meta-analysis". Biological Psychiatry. Cognitive Neuroscience and Neuroimaging. 2 (4): 327–335. doi:10.1016/j.bpsc.2017.01.011. ISSN 2451-9030. PMC 5627663. PMID 28989984.
- Richards EM, Payne JL (Oct 2013). "The management of mood disorders in pregnancy: alternatives to antidepressants". CNS Spectr (Submitted manuscript). 18 (5): 261–271. doi:10.1017/S1092852913000151. PMID 23570692. S2CID 24489076.
- Miller, Laura J. (1994). "Use of Electroconvulsive Therapy During Pregnancy". Psychiatric Services. 45 (5): 444–450. doi:10.1176/ps.45.5.444. ISSN 1075-2730. PMID 8045538.
- Duma A, Maleczek M, Panjikaran B, Herkner H, Karrison T, Nagele P (2019). "Major Adverse Cardiac Events and Mortality Associated with Electroconvulsive Therapy: A Systematic Review and Meta-analysis". Anesthesiology. 130 (1): 83–91. doi:10.1097/ALN.0000000000002488. PMC 6300062. PMID 30557212.
- Prudic J, Olfson M, Sackeim HA (July 2001). "Electro-convulsive therapy practices in the community". Psychol Med. 31 (5): 929–934. doi:10.1017/S0033291701003750. PMID 11459391. S2CID 12210381.
- Dr.Barnes, Richard. "Information on ECT". Royal College of Psychiatrists' Special Committee on ECT and related treatment. Retrieved 3 November 2013.
- Royal College of Psychiatrists. Council Report. The ECT Handbook: The Third Report of the Royal College of Psychiatrists' Special Committee of ECT. RCPsych Publications, 2005 ISBN 978-1904671220
- Duffett R, Lelliott P (1998). "Auditing electroconvulsive therapy. The third cycle". Br J Psychiatry. 172 (5): 401–405. doi:10.1192/bjp.172.5.401. PMID 9747401. S2CID 23584054.
- Lock, T (1995). "Stimulus dosing". In C Freeman (ed.) The ECT Handbook. London: Royal College of Psychiatrists, 72–87.
- Motohashi N, Awata S, Higuchi T (2004). "A questionnaire survey of ECT practice in university hospitals and national hospitals in Japan". J ECT. 20 (1): 21–23. doi:10.1097/00124509-200403000-00005. PMID 15087992. S2CID 41654261.
- Chanpattana W, Kunigiri G, Kramer BA, Gangadhar BN (2005). "Survey of the practice of electroconvulsive therapy in teaching hospitals in India". J ECT. 21 (2): 100–104. doi:10.1097/01.yct.0000166634.73555.e6. PMID 15905751. S2CID 5985564.
- Ikeji OC, Ohaeri JU, Osahon RO, Agidee RO (1999). "Naturalistic comparative study of outcome and cognitive effects of unmodified electro-convulsive therapy in schizophrenia, mania and severe depression in Nigeria". East Afr Med J. 76 (11): 644–50. PMID 10734527.
- Teena Thacker for Indian Express. Mar 23 2011 Electroshocks for mentally ill patients to be banned
- Kala, A (2013). "Time to face new realities; mental health care bill-2013". Indian Journal of Psychiatry. 55 (3): 216–219. doi:10.4103/0019-5545.117129. PMC 3777341. PMID 24082240.
- "Abusive practice of "unmodified" electroshock treatment abolished at main psychiatric facility of Turkey". Disabled Peoples' International. Archived from the original on 2007-10-12. Retrieved 2008-03-25.
- Narang, P.; Swenson, A.; Lippmann, S. (2019). "The Journal of ECT". LWW. 35 (1): e5–e6. doi:10.1097/YCT.0000000000000515. PMID 29944607. S2CID 49432743. Retrieved 2021-07-02.
- Haskett, R. F.; Loo, C. (2010). "Role of Adjunctive Psychotropic Medications during ECT in the Treatment of Depression, Mania and Schizophrenia". The Journal of ECT. 26 (3): 196–201. doi:10.1097/YCT.0b013e3181eee13f. PMC 2952444. PMID 20805728.
- Madhavan Seshadri; Nadeem Z Mazi-Kotwal. "Response Predictors in ECT: A discussion about Seizure Threshold". British Journal of Medical Practitioners. Retrieved 23 March 2016.
- Corinne Slusher for MedScape. Updated: Jan 6, 2012 Electroconvulsive Therapy Machine
- "Ectron: Our story". Archived from the original on 2014-10-27. Retrieved 2015-01-07.
- Tsoukalas Ioannis (2020). "How does ECT work? A new explanatory model and suggestions for non-convulsive applications". Medical Hypotheses. 145: 110337. doi:10.1016/j.mehy.2020.110337. PMID 33099256. S2CID 225063736.
- Leiknes, K. A.; Jarosh-von Schweder, L.; Høie, B. (2012). "Contemporary use and practice of electroconvulsive therapy worldwide". Brain and Behavior. 2 (3): 283–344. doi:10.1002/brb3.37. PMC 3381633. PMID 22741102.
- See the Slovenian government website Archived 2007-08-08 at the Wayback Machine for information about ECT in Slovenia.
- Reid WH, Keller S, Leatherman M, Mason M (January 1998). "ECT in Texas: 19 months of mandatory reporting". J Clin Psychiatry. 59 (1): 8–13. doi:10.4088/JCP.v59n0103. PMID 9491059.
- Euba R, Saiz A (2006). "A comparison of the ethnic distribution in the depressed inpatient population and in the electroconvulsive therapy clinic". J ECT. 22 (4): 235–236. doi:10.1097/01.yct.0000235928.39279.52. PMID 17143151. S2CID 28261416.
- Rise In Electric Shock Therapy In County. Archived 2011-05-01 at the Wayback Machine Sarah Hall, Norwich Evening News 24, June 4, 2008. Accessed: June 4, 2008.
- Hermann R, Dorwart R, Hoover C, Brody J (1995). "Variation in ECT use in the United States". Am J Psychiatry. 152 (6): 869–875. doi:10.1176/ajp.152.6.869. PMID 7755116.
- Cauchon, Dennis (1995-12-06). "Patients often aren't informed of full danger". USA Today. Archived from the original on 2008-01-15. Retrieved 2008-01-01.
- "Electroconvulsive Therapy in Children | Psychology Today". www.psychologytoday.com.
- Texas Department of State (2002) Electroconvulsive therapy reports Archived 2007-08-10 at the Wayback Machine.
- Fink, M. & Taylor, A.M. (2007) "Electroconvulsive therapy: Evidence and Challenges" JAMA Vol. 298 No. 3, pp. 330–332.
- Pippard J, Ellam L (1981). "Electroconvulsion treatment in Great Britain 1980". Lancet. 2 (8256): 1160–1161. doi:10.1016/s0140-6736(81)90602-4. PMID 6118592. S2CID 30499609.
- Electro convulsive therapy: survey covering the period from January 2002 to March 2002. Department of Health.
- NICE 2003. Electroconvulsive therapy (ECT). Retrieved on 2007-12-29.
- Carney, S; Geddes, J (2003). "Electroconvulsive therapy: recent recommendations are likely to improve standards and uniformity of use". British Medical Journal. 326 (7403): 1343–1344. doi:10.1136/bmj.326.7403.1343. PMC 1126234. PMID 12816798.
- NICE (2003). Appraisal of electroconvulsive therapy: decision of the appeal panel Archived 2007-05-21 at the Wayback Machine. London: NICE.
- Duffett, R; Lelliott, P (1998). "Auditing electroconvulsive therapy: the third cycle". British Journal of Psychiatry. 172 (5): 401–405. doi:10.1192/bjp.172.5.401. PMID 9747401. S2CID 23584054.
- Royal College of Psychiatrists (2017). 2016–2017.
- "Additional safeguards for ECT introduced in new s58A – Mental Health Law Online".
- Tang YL, et al. (Dec 2012). "Electroconvulsive therapy in China: clinical practice and research on efficacy". J ECT. 28 (4): 206–212. doi:10.1097/YCT.0b013e31825957b1. PMID 22801297. S2CID 2743272.
- Graham-Harrison, Emma; Connaire, Shaunagh (8 October 2015). "Chinese hospitals still offering gay 'cure' therapy, film reveals". The Guardian.
- Hannah Beech (June 13, 2016). "This Man Was Sectioned in China for Being Gay. Now He's Fighting Back". Time. Retrieved October 20, 2017.
- Stone, R. (2009-07-26). "China Reins in Wilder Impulses in Treatment of 'Internet Addiction'". Science. 324 (5935): 1630–1631. Bibcode:2009Sci...324.1630S. doi:10.1126/science.324_1630. PMID 19556477.
- A History of Mental Institutions in the United States which says electrostatic machines were used in 1773
- "Lind, James (1736–1812) on JSTOR". plants.jstor.org. Retrieved 2021-05-08.
- Parent, Andre (2004). "Giovanni Aldini: from animal electricity to human brain stimulation". Can J Neurol Sci. 31 (4): 576–584. doi:10.1017/s0317167100003851. PMID 15595271.
- Wright, Bruce A. M.D. "An Historical Review of Electro Convulsive Therapy". Jefferson Journal of Psychiatry: 66–74.
- Beveridge, A. W.; Renvoize, E. B. (1988). "Electricity: A History of its use in the Treatment of Mental Illness in Britain During the Second Half of the 19th Century" (PDF). British Journal of Psychiatry. 153 (2): 157–162. doi:10.1192/bjp.153.2.157. PMID 3076490. S2CID 31015334. Archived from the original (PDF) on 23 September 2015. Retrieved 28 December 2014.
- Berrios, G E (1997). "The scientific origins of electroconvulsive therapy". History of Psychiatry. 8 (29 pt 1): 105–119. doi:10.1177/0957154X9700802908. PMID 11619203. S2CID 12121233.
- Fink, M (1984). "The origins of convulsive therapy". American Journal of Psychiatry. 141 (9): 1034–1041. doi:10.1176/ajp.141.9.1034. PMID 6147103.
- Bolwig, T. (2011). "How does electroconvulsive therapy work? Theories on its mechanism". The Canadian Journal of Psychiatry. 56 (1): 13–18. doi:10.1177/070674371105600104. PMID 21324238.
- Bangen, Hans: Geschichte der medikamentösen Therapie der Schizophrenie. Berlin, 1992, ISBN 3927408824
- Sabbatini, R. "The history of shock therapy in psychiatry". Retrieved 2013-04-24.
- Cerletti, U (1956). "Electroshock therapy". In AM Sackler et al. (eds) The Great Physiodynamic Therapies in Psychiatry: an historical appraisal. New York: Hoeber-Harper, 91–120.
- Sirgiovanni, Elisabetta; Aruta, Alessandro (April 23, 2020). "From the Madhouse to the Docu-Museum: The Enigma Surrounding the Cerletti-Bini ECT Apparatus Prototype". Nuncius. 35 (1): 141. doi:10.1163/18253911-03501013. S2CID 218991982.
- Sirgiovanni, E, Aruta, A (2020) "The Electroshock Triangle: Disputes about the ECT Apparatus Prototype and its Display in the 1960s, History of Psychiatry. First Published April 20, 2020: https://doi.org/10.1177/0957154X20916147.
- Kiloh, LG, Smith, JS, Johnson, GF (1988). Physical Treatments in Psychiatry. Melbourne: Blackwell Scientific Publications, 190–208. ISBN 0867931124
- Goode, Erica (1999-10-06). "Federal Report Praising Electroshock Stirs Uproar". The New York Times. Retrieved 2008-01-01.
- Goleman, Daniel (1990-08-02). "The Quiet Comeback of Electroshock Therapy". The New York Times. p. B5. Retrieved 2008-01-01.
- See:
- Friedberg, J (1977). "Shock treatment, brain damage, and memory loss: a neurological perspective". American Journal of Psychiatry. American Psychiatric Association Publishing. 134 (9): 1010–1014. doi:10.1176/ajp.134.9.1010. ISSN 0002-953X. PMID 900284.
- Breggin, PR (1979). Electroshock: its brain-disabling effects. New York: Springer. ISBN 082612710X. OCLC 5029460.
- Blaine, JD; Clark, SM (1986). "Report of the NIMH–NIH consensus development conference on Electroconvulsive therapy". Psychopharmacology Bulletin. 22 (2): 445–452. PMID 3774937.
- Dutton, Audrey (2017-02-18). "This mental health treatment isn't barbaric, it 'totally changed my life'".
- "Electroconvulsive therapy: How modern techniques improve patient outcomes".
- Hiroaki I, Hirohiko H, Masanari I (2012). "A case of schizophrenia successfully treated by m-ECT using 'long' brief pulse". International Journal of Case Reports and Images. 3 (7): 30. doi:10.5348/ijcri-2012-07-147-CR-8.
- Fisher P (Dec 2012). "Psychological factors related to the experience of and reaction to electroconvulsive therapy". J Ment Health. 21 (6): 589–599. doi:10.3109/09638237.2012.734656. PMID 23216225. S2CID 42581352.
- Philpot, M; Treloar, A; Gormley, N; Gustafson, L (2002). "Barriers to the use of electroconvulsive therapy in the elderly: a European survey". European Psychiatry. 17 (1): 41–45. doi:10.1016/S0924-9338(02)00620-X. PMID 11918992. S2CID 24740314.
- Whitaker, Robert (2010). Mad in America: bad science, bad medicine, and the enduring mistreatment of the mentally ill (Rev. pbk. ed.). New York: Basic Books. pp. 102–106. ISBN 978-0465020140.
- Golenkov, A.; Ungvari, G. S.; Gazdag, G. (21 February 2011). "Public attitudes towards electroconvulsive therapy in the Chuvash Republic". International Journal of Social Psychiatry. 58 (3): 289–294. doi:10.1177/0020764010394282. PMID 21339235. S2CID 6300979.
- Committee on Mental Health (March 2002). "Report on Electroconvulsive Therapy". New York State Assembly. Archived from the original on 30 April 2011. Retrieved 8 March 2011.
- Melding, P (2006-07-07). "Electroconvulsive therapy in New Zealand: terrifying or electrifying?". The New Zealand Medical Journal. 119 (1237): U2051. PMID 16862197. Archived from the original on 2011-05-01. Retrieved 2011-03-08.
- Teh, S.P.C.; Helmes, E.; Drake, D. (2007). "A Western Australian Survey On Public Attitudes Toward and Knowledge of Electroconvulsive Therapy". International Journal of Social Psychiatry. 53 (3): 247–271. doi:10.1177/0020764006074522. PMID 17569409. S2CID 40147979.
- Kellner, Charles H. (2012-07-05). "The FDA Advisory Panel on the Reclassification of ECT Devices: Unjustified Ambivalence". Psychiatric Times. UBM Medica. Archived from the original on 2012-08-21. Retrieved 2012-10-25.
- Duff Wilson for the New York Times. January 28, 2011 F.D.A. Panel Is Split on Electroshock Risks
- Mechcatie, Elizabeth. "FDA Regulation of ECT Devices in Transition". Clinical Psychiatry News. Retrieved 8 March 2011.
- Fergusson G, et al., eds. (2009). "The Scottish ECT Accreditation Network (SEÁN) Annual Report 2009" (PDF). Scottish ECT Accreditation Network. Retrieved 2010-05-24.
- Jones, R (1996) Mental Health Act Manual, 5th edition. London: Sweet and Maxwell, p. 225.
- Rose D, Wykes T, Bindman J, Fleischmann P (2005)"Information, consent and perceived coercion: patients' perspectives on electroconvulsive therapy". British Journal of Psychiatry 186:54–59.
- Lutchman, RD; et al. (2001). "Mental health professionals' attitudes towards and knowledge of electroconvulsive therapy". Journal of Mental Health. 10 (20): 141–150. doi:10.1080/09638230124779. S2CID 218906587.
- The Mental Health Act 1983 (updated version) Archived December 26, 2011, at the Wayback Machine Part IV, Section 58. Care Quality Commission
- Care Quality Commission (2010) ECT: Your rights about consent to treatment
- The Mental Health Act 1983 (updated version) Part IV, Section 62. Care Quality Commission
- The Mental Health Act Commission (2005) In Place of Fear? eleventh biennial report, 2003–2005, 236. The Stationery Office.
- "CQC says care for people treated under the Mental Health Act still needs to improve". Care Quality Commission. 8 December 2011. Archived from the original on 21 May 2015.
- The Mental Health (Care and Treatment) (Scotland) Act 2003, Part 16, sections 237–239.
- Levin, Saul, M.D., M.P.A.; Binder, Renée M.D. "Time Is Now to Support the ECT Reclassification Effort". American Psychiatric Association. Retrieved 23 April 2017.
- McFarquhar, Tara F.; Thompson, James (December 2008). "Knowledge and Attitudes Regarding Electroconvulsive Therapy Among Medical Students and the General Public". The Journal of ECT. 24 (4): 244–253. doi:10.1097/YCT.0b013e318168be4a. PMID 18648319. S2CID 11334694.
- Smith, Daniel (2001-02-01). "Shock and Disbelief". Atlantic Monthly. 287 (2): 79–90. PMID 15997536. Retrieved 2019-12-30.
- A. E. Hotchner, Papa Hemingway: A Personal Memoir, ISBN 0786705922; p. 280
- "All About Heaven \e". allaboutheaven.org. Retrieved 2019-12-30.
- Worth Books (2017). Summary and Analysis of Zen and the Art of Motorcycle Maintenance: An Inquiry into Values. Open Road Media. ISBN 978-1504046411.
- Healy, David; Charlton, Bruce G. (May 2009). "Electroshock in Zen and the Art of Motorcycle Maintenance – fictional, not factual". Medical Hypotheses. 72 (5): 485–486. doi:10.1016/j.mehy.2008.12.026. ISSN 1532-2777. PMID 19201545.
- "Sherwin Nuland: How electroshock therapy changed me | Talk Subtitles and Transcript". TED.com. 30 October 2007. Retrieved 2015-05-19.
- Gellene, Denise (2014-03-04). "Sherwin B. Nuland, Author of 'How We Die,' is Dead at 83". The New York Times.
- Lipsky, Dave (October 30, 2008). "The Lost Years & Last Days of David Foster Wallace". Rolling Stone. Archived from the original on May 3, 2009. Retrieved June 5, 2017.
- Lim, Xinhui; Galletly, Cherrie (4 April 2019). ""To suit the occasion, I wore my schizophrenic fancy dress" – the life of Janet Frame". Australasian Psychiatry. 27 (5): 469–471. doi:10.1177/1039856219839489. PMID 30945930. S2CID 93000402.
- "Review/Film; 3 Novels Are Adapted For 'Angel at My Table'". The New York Times. 21 May 1991. Section C, p. 15. Retrieved 10 July 2020.
- "Wishful Drinking with Carrie Fisher". NPR.
- Kellner C.H. (2013). Electroconvulsive Therapy (ECT) in Literature: Sylvia Plath's 'The Bell Jar'. Prog. Brain Res. Progress in Brain Research. Vol. 206. pp. 219–228. doi:10.1016/B978-0-444-63364-4.00029-6. ISBN 978-0444633644. PMID 24290484.
- Mitchell & Snyder, p. 174
- "Report of the Electro-convulsive Therapy Review Committee." Toronto: Electro-convulsive Therapy Review Committee, 1985.
- Grosser G (1975). "The Regulation of Electroshock Treatment in Massachusetts". Massachusetts Journal of Mental Health. 5: 12–25.
- Weitz, D. "Ontario Electroshock Statistics." Figures released under the Freedom of Information Act. Toronto: Ontario. Ministry of Health, 2001.
External links
- Position Statement on Electroconvulsive Therapy (ECT) 2015 – from the American Psychiatric Association.
- ECT – information from mental health charity The Royal College of Psychiatrists