Corynebacterium pseudotuberculosis
Corynebacterium pseudotuberculosis is a Gram-positive bacterium known globally to infect ruminants, horses, and rarely people. This bacterium is a facultative anaerobic organism that is catalase positive and capable of beta-hemolysis. In small ruminants, C. pseudotuberculosis causes a disease called caseous lymphadenitis characterized by pyogranulomatous abscess formation. In general, this bacterium causes lesions of the skin, lymph nodes, and internal organs. A disease known as ulcerative lymphagenitis can also result from infection with C. pseudotuberculosis in the distal limbs of horses. This bacterium uses the virulence factors phospholipase D and mycolic acid to damage eukaryotic cell walls and resist phagocytic lysosomal degradation, respectively. Infection with this bacterium is often confirmed by bacterial culture of the purulent exudate. Once the diagnosis has been made, treatment of the infection can begin, but this is difficult due to the nature of the organism and the lesions it forms. Specifically, C. pseudotuberculosis is intrinsically resistant to streptomycin, with varying resistance to penicillin and neomycin depending on the strain. It has been shown to be susceptible to ampicillin, gentamicin, tetracycline, lincomycin, and chloramphenicol. Vaccines have also been produced to develop acquired immunity to this infection.
| Corynebacterium pseudotuberculosis | |
|---|---|
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Actinomycetota |
| Class: | Actinomycetia |
| Order: | Mycobacteriales |
| Family: | Corynebacteriaceae |
| Genus: | Corynebacterium |
| Species: | C. pseudotuberculosis |
| Binomial name | |
| Corynebacterium pseudotuberculosis (Buchanan 1911) Eberson 1918 (Approved Lists 1980) | |
History, epidemiology and zoonotic risk
The first isolation of C. pseudotuberculosis came from a cow lymph node in 1888, by French bacteriologist Edmond Nocard. Shortly after, it was sampled from abscesses in a sheep by Hugo von Preisz, and the bacterium was named the ‘‘Preisz-Nocard’’ bacillus until further work by German microbiologists in the mid 1900s, when it was recategorized into the Corynebacterium genus. It was finally renamed Corynebacterium pseudotuberculosis in 1948, to reflect that the clinical disease signs were similar to disease cause by M. tuberculosis species.
Distribution of C. pseudotuberculosis is mostly traced by examining prevalence of caseous lymphadenitis, the bacterium’s main pathological disease. Infection of domesticated sheep and goats has been found across the globe. Although few recent studies have been conducted into its prevalence, data from slaughterhouses in Australia in the late 1980s suggested that C. pseudotuberculosis was affecting 50–60% of sheep. Development of a vaccine was completed in 1983, and it was added to the recommended clostridial vaccines for sheep.[1] The national average of prevalence in Australia was 5.2% in 2009, although this varies by region.[1] Prevalence in Canadian sheep, mainly based on numbers from Alberta and Quebec abattoirs, has been reported between 8 and 36%. C. pseudotuberculosis also causes disease in horses, and should be considered prevalent in areas where cases of "pigeon fever" and "ulcerative lymphadenitis" have been recorded.[2]
This disease is spread between infected animals by vectors, and through contamination of the environment with exudate from abscesses. Major bacterial spread occurs when superficial abscesses are broken open and release discharge that is then contacted by other animals during grooming, or contaminates feed, water, and bedding that other animals consume.[2] These abscesses may break open spontaneously or be broken open on surfaces or during shearing. C. pseudotuberculosis can survive in soil for up to 8 months and contaminate bedding and indoor handling facilities for several weeks. In horses, arthropod vectors are considered a significant source of infection.[2] As vector patterns change with warming temperatures, C. pseudotuberculosis in horses is re-emerging in the United States.[2]
This bacterial species has caused occasional cases of infection in people who work closely with infected small ruminants, resulting in similar swellings of the lymph nodes in the neck and groin.[3][1] The most likely route of infection is direct contact with the infected animal, or its raw products.[3] Therefore, mostly farm workers and animal health workers are at risk.[1]
Cellular morphology, biochemistry, and identification
C. pseudotuberculosis is a Gram-positive bacterium that can be either coccoid or filamentous rods, which can be organized into palisades.[4] Metachromatic volutin granules containing phosphate can be seen in the rod form, but not the coccoid form when stained using Albert's or Neisser's methods.[4] Other characteristics of this bacterium include being nonsporulating, noncapsulated, and possessing fimbrae, but it is immobile.[4]
This bacterium is grown at 37 °C under aerobic or anaerobic conditions, thus C. pseudotuberculosis is a facultative anaerobe.[5] It forms dry, pale yellow colonies measuring 1–2 mm in diameter after incubation on solid media for 48 hours.[5] Media containing serum or whole blood improve bacterial growth and a band of beta-hemolysis tends to forms around bacterial colonies after 48–72 hours of incubation on blood agar.[5] C. pseudotuberculosis forms clumps in fluid media.[5]
While strains of C. pseudotuberculosis are consistent in their morphology and growth on media, they show greater variation in biochemical properties such as fermenting ability. While they are unable to produce gas, all strains can use glucose, fructose, maltose, mannose, and sucrose to produce acid.[4] Additional biochemical properties of this bacterium include being phospholipase D and positive for catalase, oxidase negative, and beta-hemolytic.[4] Generally, two subtypes of C. pseudotuberculosis are known; the equi biovar affects horses and cattle, while the ovis biovar affects small ruminants.[6] Their ability to reduce nitrate can be used to distinguish between the subtypes. The equi biovar can reduce nitrate, while the ovis biovar generally cannot, but some exceptions have been demonstrated.[7] Coryneform bacteria, including C. pseudotuberculosis, can also be differentiated using a biochemical test called the API Coryne system, which involves enzyme and carbohydrate fermentation tests and requires 24–48 hours to perform.[4] Finally, genetic sequence analysis can be used to confirm the identification of C. pseudotuberculosis if biochemical identification is not sufficient.[6] In fact, a multiplex polymerase chain reaction assay has been developed using a number of characteristic genes that can differentiate between the closely related species of corynebacteria - C. pseudotuberculosis, C. ulcerans, and C. diphtheriae.[8]
Clinical signs
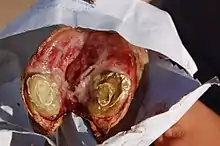
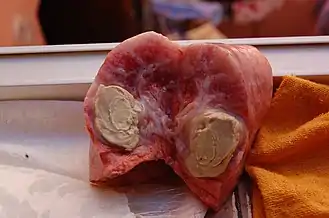
Disease in small ruminants
C. pseudotuberculosis causes a disease known as caseous lymphadenitis that most commonly affects small ruminants, such as goats and sheep.[9][10] Caseous lymphadenitis often presents with pyogranulomatous abscess formation.[11] Abscessation can occur in numerous areas, but it most commonly affects the cutaneous region and superficial lymph nodes. This is known as the external form of the disease and is the primary form affecting goats. Nodular lesions are often visible. The internal form of the disease is more of a concern for sheep. In this form, internal organs and lymph nodes are affected.[10] Over time, these abscesses deposit multiple layers of fibrous capsules which gives them a lamellated appearance upon cross section.[11][10] The contents of the abscess are thick and purulent, and the abscess often ruptures. This can be an important source of contamination to other animals in the herd.[11]
The internal manifestation of the disease is harder to recognize, as the clinical signs are not as obvious; however, they may include a reduction in reproductive ability and diminished body condition.[12] Depending on the location of the lesions and the pressure they exert on their surroundings, the animal may develop dysphagia and abnormal rumination.[11]
Disease in cattle
The most common clinical sign for infected cattle is ulcerative lesions of the skin.[13] Mastitis, an infection of the udder that is most commonly caused by species of the Streptococcus and Staphylococcus genera, may also be caused by C. pseudotuberculosis in rare circumstances.[14][11][13] Infected cattle may also have a higher frequency of abortions.[13]
Disease in horses
C. pseudotuberculosis can also cause disease in horses, which also present with abscessation, but the lesions are most commonly seen on the underside of the abdomenal and pectoral regions. C. pseudotuberculosis can also cause infection of the distal limbs, a manifestation known as ulcerative lymphangitis. It is usually a unilateral lesion and the affected limb is painful, swollen, and contains draining ulcerative and nodular lesions.[13]
Diagnosis
Diagnosis of C. pseudotuberculosis can be difficult due to vague clinical signs such as weight loss and general ill thrift.[12] To confirm infection in animals with the external form, a bacteriological culture of the purulent material from an intact abscess should be taken. Biopsies may also be useful. If C. pseudotuberculosis is isolated, then this provides definitive diagnosis.[13] Confirming diagnosis in animals infected with the internal form of the disease is more difficult, but ultrasonography and/or radiography may be useful.[10]PCR has shown promising results for diagnosis and a double-antibody sandwich ELISA can be used in sheep and goats.[12]
Pathogenicity and virulence
C. pseudoteburculosis has two well documented virulence factors, phospholipase D (an endotoxin),[15][16][17] and a mycolic acid surface lipid.[15] Both virulence factors are proposed to be independent of plasmids, as no plasmid has been found found in C. pseudoteburculosis.[15]
Phospholipase D
Every isolate of C. pseudoteburculosis in research so far has had the phospholipase D virulence factor.[15] Studies that examine the absence or mutation of the phosphlipase D gene in C. pseudoteburculosis-infected mice have been shown to fail to develop chronic abscessation.[16] This phospholipase encoding gene has been isolated to pathogenicity island 1.[18] At least seven pathogenicity islands have been recorded in C. pseudoteburculosis.[18]
Phospholipase D is an ester bond-cleaving exotoxin that provides the bacterium with the ability to cleave sphingomyelin,[15] which is a glycerophospholipid eukaryotic cell-wall component,[19] so by destruction or cleavage of this component, the cell wall becomes damaged.[15] This can cause death or loss of function of eukaryotic cells. This effect may help the invasion of C. pseudoteburculosis.[18]
Phospholipase D also increases vascular permeability,[15][17] possibly due to sphingomyelinase effects. The increase in permeability may allow increased lymphatic drainage, leading to one of the mechanisms of migration to the lymph nodes, where C. pseudoteburculosis causes chronic abscessation.[17]
Mycolic acid
Another mechanism of lymphatic transport is facilitated by the presence of a mycolic acid surface lipid. This coats the C. pseudoteburculosis organisms.[15] Mycolic acid provides a protective barrier, allowing the bacterial cell to resist lysosomal degradation by eukaryotic, phagocytic, white blood cells.[17][15] This allows the bacteria to act as facultative intracellular parasites once they have been phagocytized, where the phagocyte eventually migrates to the lymph node, where chronic infection occurs.[15] Furthermore, the surface lipid is cytotoxic and can cause death to macrophages.[15]
In a prospective study involving 12 Boer goats, inoculation with mycolic acid compared to control animals provided statistically significant evidence of pathological changes in the lung compared to a control group.[20] Groups inoculated with mycolic acid or C. pseudoteburculosis were evaluated through histopathology compared to a control. Both groups showed haemorrhage, congestion, oedema, inflammation, and necrosis. Organs affected by these degenerative changes included the lung, heart, kidney, and spleen, though severity varied within organs and within mycolic only, and C. pseudoteburculosis-infected groups.[20] The importance of this virulence factor has also been highlighted where mice that have been injected with mycolic acid were shown to produce a chronic abscessation that increased with higher doses.[15]
Differences in strain genomics
Four different strains of C. pseudoteburculosis are recognized: Cp1002, CpC231, Cp119, and CpFRC41.[18] Comparing genes within these strains, the largest genome was found to be 2377 genes, of which 1851 genes were shared among all four strains.[18] In a CpFRC41 strain isolated from a girl, a gene was found to encode for superoxide dismutase among other specific virulence factors.[21] Superoxide dismutase is involved in evading the immune system by deactivating reactive chemicals secreted by the body that would otherwise kill the bacterial cell.[22] When comparing strains Cp1002 and CpC231 in the sixth pathogenictiy island (PiCp6), Cp1002 contained the gene pipA1, where CpC231 contained pipB.[18] This island (6) codes for proline iminopeptidase, which is involved with removal of proline from proteins.
Of great importance, only a small portion of the documented virulence factors have been listed here. Through genomic evaluation, many more possible virulence factors have been found.[18][21][22]
Treatment
Antimicrobial Therapy
Treatment of C. pseudotuberculosis in infected animals has been proven difficult, primarily due to the nature of the lesions and the facultative intracellular nature of the organism.[15][23] Strains of C. pseudotuberculosis have shown to be susceptible to numerous antimicrobial therapies in vitro, including ampicillin, gentamicin, tetracycline, lincomycin, chloramphenicol, and others. Treatment within live animals (in vivo) is thought to be limited due to the firm capsule and thick, caseous nature of the abscess lesions, which make them a difficult target for antimicrobial therapy.[15][24] Most strains of C. pseudotuberculosis have been shown to be intrinsically resistant to streptomycin, with varying resistance to penicillin and neomycin depending on the strain.[24]
Vaccination
Several vaccine types are currently available for treatment of C. pseudotuberculosis infections, including bacterin vaccines, toxoid vaccines, combined vaccines, live vaccines, and DNA vaccines.[17] Part of the C. pseudotuberculosis lifecycle being intracellular adds an additional element of difficulty to vaccine treatment, as a vaccine must be able to induce a cell-mediated response rather than a solely humoral antibody response to eliminate the bacteria from the body.[23] A vaccine using a recombinant form of the phospholipase D exotoxin is now in widespread use, and has been shown to decrease the occurrence of lung lesions and amount of tissue damage observed upon infection.[1] Though vaccination will not prevent infection in a flock, it does minimize the severity of infection, so can have positive implications in reducing carcass condemnations for producers.[1] This exotoxin is commonly included in combination vaccines that also protect against clostridial diseases.[1] Good management practices that include the implementation of persistent vaccination protocols with the combination vaccine Glanvac 6 (Zoetis) have demonstrated to be quite effective, and have led to a decrease of caseous lymphadenitis in Australia.[1] Vaccines should be administered as directed by the manufacturer for highest efficacy, ensuring that the vaccines are given to the appropriate animals at the correct ages, and are boosted at the recommended intervals.[17]
Husbandry
With antimicrobial therapy and vaccination protocols being somewhat limited, proper management practices are highly recommended for controlling caseous lymphadenitis.[25] Good husbandry practices can decrease the occurrence and spread of caseous lymphadenitis among small ruminants, thus improving animal health and minimizing economic impacts on the producers.[23] C. pseudotuberculosis is commonly spread through superficial wounds inflicted during shearing, castration, tail docking, and ear tagging, so disinfection of equipment between animals and keeping wound sites clean can help limit spread.[23] The disease can also spread through superficial wounds caused during animal fighting, so care should be taken to minimize aggression among animals.[23] C. pseudotuberculosis can persist on fomites such as straw bedding, hay, wood, and feces for weeks, and in the soil for up to 8 months, making proper disinfection of facilities and pasture management essential to limit spread of disease.[23] In addition, all animals should be closely monitored for any lesions or signs of disease to remove infected animals from the flock or herd as early as possible.[25] Infected animals should be quarantined, and in some cases, culling of infected animals may be indicated.[23]
References
- Windsor, P. A.; Bush, R. D. (2016-09-01). "Caseous lymphadenitis: Present and near forgotten from persistent vaccination?". Small Ruminant Research. Special Issue: Keynote Lectures of The XL National congress and XVI International of The Spanish Society for Sheep and Goat Production (S.E.O.C.). 142: 6–10. doi:10.1016/j.smallrumres.2016.03.023. ISSN 0921-4488.
- Spier, S. J.; Azevedo, V. (2017-08-09). "Corynebacterium pseudotuberculosis infection in horses: Increasing frequency and spread to new regions of North America". Equine Veterinary Education. 29 (8): 436–439. doi:10.1111/eve.12589.
- Bauerfeind, R. (Rolf) (December 2015). Zoonoses. Krauss, H. (Fourth ed.). Washington, D.C. ISBN 978-1-68367-332-3. OCLC 1147868070.
- Dorella, Fernanda Alves; Carvalho Pacheco, L.; Oliveira, Sergio Costa; Miyoshi, Anderson; Azevedo, Vasco (2006). "Corynebacterium pseudotuberculosis: microbiology, biochemical properties, pathogenesis and molecular studies of virulence". Veterinary Research. 37 (2): 201–218. doi:10.1051/vetres:2005056. ISSN 0928-4249. PMID 16472520.
- Baird, G.J.; Fontaine, M.C. (2007). "Corynebacterium pseudotuberculosis and its Role in Ovine Caseous Lymphadenitis". Journal of Comparative Pathology. 137 (4): 179–210. doi:10.1016/j.jcpa.2007.07.002. ISSN 0021-9975. PMID 17826790.
- Domenis, L.; Spedicato, R.; Pepe, E.; Orusa, R.; Robetto, S. (May 2018). "Caseous Lymphadenitis Caused by Corynebacterium pseudotuberculosis in Alpine Chamois ( Rupicapra r. rupicapra ): a Review of 98 Cases". Journal of Comparative Pathology. 161: 11–19. doi:10.1016/j.jcpa.2018.04.003. ISSN 0021-9975. PMID 30173853.
- Williamson, Lisa H. (July 2001). "Caseous Lymphadenitis in Small Ruminants". Veterinary Clinics of North America: Food Animal Practice. 17 (2): 359–371. doi:10.1016/s0749-0720(15)30033-5. ISSN 0749-0720. PMID 11515406.
- D'Afonseca, V (2008). "Description of Genes of Corynebacterium Pseudotuberculosis Useful in Diagnostics and Vaccine Applications". Genetics and Molecular Research. GMR 7.1 (1): 252–260. doi:10.4238/vol7-1gmr438. PMID 18551390.
- Gao, Huafeng; Ma, Yuxing; Shao, Qingyong; Hong, Qionghua; Zheng, Guoying; Li, Zhiming (2018-03-15). "Genome Sequence of Corynebacterium pseudotuberculosis Strain KM01, Isolated from the Abscess of a Goat in Kunming, China". Genome Announcements. 6 (11). doi:10.1128/genomeA.00013-18. ISSN 2169-8287. PMC 5854783. PMID 29545288.
- "Caseous Lymphadenitis of Sheep and Goats – Circulatory System". Merck Veterinary Manual. Retrieved 2020-10-06.
- Fontaine, M. C.; Baird, G. J. (2008-04-01). "Caseous lymphadenitis". Small Ruminant Research. Special Issue: Current Issues in Sheep Health and Welfare. 76 (1): 42–48. doi:10.1016/j.smallrumres.2007.12.025. ISSN 0921-4488.
- Chakraborty, Sandip; Kumar, Amit; Tiwari, Ruchi; Rahal, Anu; Malik, Yash; Dhama, Kuldeep; Pal, Amar; Prasad, Minakshi (2014-06-15). "Advances in Diagnosis of Respiratory Diseases of Small Ruminants". Veterinary Medicine International. 2014: 508304. doi:10.1155/2014/508304. PMC 4082846. PMID 25028620.
- "Corynebacterium pseudotuberculosis Infection of Horses and Cattle – Circulatory System". Merck Veterinary Manual. Retrieved 2020-10-05.
- Ruegg, Pamela L. (2017-12-01). "A 100-Year Review: Mastitis detection, management, and prevention". Journal of Dairy Science. 100 (12): 10381–10397. doi:10.3168/jds.2017-13023. ISSN 0022-0302. PMID 29153171.
- Baird, G. J.; Fontaine, M. C. (2007-11-01). "Corynebacterium pseudotuberculosis and its Role in Ovine Caseous Lymphadenitis". Journal of Comparative Pathology. 137 (4): 179–210. doi:10.1016/j.jcpa.2007.07.002. ISSN 0021-9975. PMID 17826790.
- Songer, J. Glenn (1997-04-01). "Bacterial phospholipases and their role in virulence". Trends in Microbiology. 5 (4): 156–161. doi:10.1016/S0966-842X(97)01005-6. ISSN 0966-842X. PMID 9141190.
- Windsor, Peter A. (2011-03-01). "Control of Caseous Lymphadenitis". Veterinary Clinics of North America: Food Animal Practice. Therapeutics and Control of Sheep and Goat Diseases. 27 (1): 193–202. doi:10.1016/j.cvfa.2010.10.019. ISSN 0749-0720. PMID 21215903.
- Ruiz, Jerônimo C.; D'Afonseca, Vívian; Silva, Artur; Ali, Amjad; Pinto, Anne C.; Santos, Anderson R.; Rocha, Aryanne A. M. C.; Lopes, Débora O.; Dorella, Fernanda A.; Pacheco, Luis G. C.; Costa, Marcília P. (2011-04-18). Mokrousov, Igor (ed.). "Evidence for Reductive Genome Evolution and Lateral Acquisition of Virulence Functions in Two Corynebacterium pseudotuberculosis Strains". PLOS ONE. 6 (4): e18551. Bibcode:2011PLoSO...618551R. doi:10.1371/journal.pone.0018551. ISSN 1932-6203. PMC 3078919. PMID 21533164.
- Slotte, J. Peter; Ramstedt, Bodil (2007). "The functional role of sphingomyelin in cell membranes". European Journal of Lipid Science and Technology. 109 (10): 977–981. doi:10.1002/ejlt.200700024. ISSN 1438-9312.
- Odhah, Mohammed Naji; Abdullah Jesse, Faez Firdaus; Teik Chung, Eric Lim; Mahmood, Zaid; Haron, Abd Wahid; Mohd Lila, Mohd Azmi; Zamri-Saad, Mohd (2019-10-01). "Clinico-pathological responses and PCR detection of Corynebacterium pseudotuberculosis and its immunogenic mycolic acid extract in the vital organs of goats". Microbial Pathogenesis. 135: 103628. doi:10.1016/j.micpath.2019.103628. ISSN 0882-4010. PMID 31325572. S2CID 198131328.
- Trost, Eva; Ott, Lisa; Schneider, Jessica; Schröder, Jasmin; Jaenicke, Sebastian; Goesmann, Alexander; Husemann, Peter; Sto ye, Jens; Dorella, Fernanda Alves; Rocha, Flavia Souza; de Castro Soares, Siomar (December 2010). "The complete genome sequence of Corynebacterium pseudotuberculosis FRC41 isolated from a 12-year-old girl with necrotizing lymphadenitis reveals insights into gene-regulatory networks contributing to virulence". BMC Genomics. 11 (1): 728. doi:10.1186/1471-2164-11-728. ISSN 1471-2164. PMC 3022926. PMID 21192786.
- Santana-Jorge, Karina T. O.; Santos, Túlio M.; Tartaglia, Natayme R.; Aguiar, Edgar L.; Souza, Renata F. S.; Mariutti, Ricardo B.; Eberle, Raphael J.; Arni, Raghuvir K.; Portela, Ricardo W.; Meyer, Roberto; Azevedo, Vasco (December 2016). "Putative virulence factors of Corynebacterium pseudotuberculosis FRC41: vaccine potential and protein expression". Microbial Cell Factories. 15 (1): 83. doi:10.1186/s12934-016-0479-6. ISSN 1475-2859. PMC 4869379. PMID 27184574.
- Williamson, Lisa H. (2001-07-01). "Caseous Lymphadenitis in Small Ruminants". Veterinary Clinics of North America: Food Animal Practice. 17 (2): 359–371. doi:10.1016/S0749-0720(15)30033-5. ISSN 0749-0720. PMID 11515406.
- Dorella, Fernanda Alves; Pacheco, Luis Gustavo Carvalho; Oliveira, Sergio Costa; Miyoshi, Anderson; Azevedo, Vasco (2006-03-01). "Corynebacterium pseudotuberculosis: microbiology, biochemical properties, pathogenesis and molecular studies of virulence". Veterinary Research. 37 (2): 201–218. doi:10.1051/vetres:2005056. ISSN 0928-4249. PMID 16472520.
- Dorella, Fernanda A.; Pacheco, Luis GC; Seyffert, Núbia; Portela, Ricardo W.; Meyer, Roberto; Miyoshi, Anderson; Azevedo, Vasco (2009-02-01). "Antigens of Corynebacterium pseudotuberculosis and prospects for vaccine development". Expert Review of Vaccines. 8 (2): 205–213. doi:10.1586/14760584.8.2.205. ISSN 1476-0584. PMID 19196200. S2CID 19391961.