Dentate gyrus
The dentate gyrus (DG) is part of the hippocampal formation in the temporal lobe of the brain, which also includes the hippocampus and the subiculum. The dentate gyrus is part of the hippocampal trisynaptic circuit and is thought to contribute to the formation of new episodic memories,[1][2] the spontaneous exploration of novel environments[2] and other functions.[3]
| Dentate gyrus | |
|---|---|
 Diagram of hippocampal regions. DG: Dentate gyrus. | |
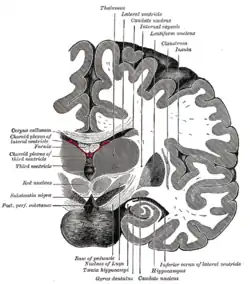 Coronal section of brain immediately in front of pons. (Label for "Gyrus dentatus" is at bottom center.) | |
| Details | |
| Part of | Temporal lobe |
| Artery | Posterior cerebral Anterior choroidal |
| Identifiers | |
| Latin | gyrus dentatus |
| MeSH | D018891 |
| NeuroNames | 179 |
| NeuroLex ID | birnlex_1178 |
| TA98 | A14.1.09.237 A14.1.09.339 |
| TA2 | 5521 |
| FMA | 61922 |
| Anatomical terms of neuroanatomy | |
It is notable as being one of a select few brain structures known to have significant rates of adult neurogenesis in many species of mammals, from rodents to primates.[4] Other sites of adult neurogenesis include the subventricular zone, the striatum[5] and the cerebellum.[6] However, whether significant neurogenesis exists in the adult human dentate gyrus has been a matter of debate.[7][8] 2019 evidence has shown that adult neurogenesis does take place in the subventricular zone and in the subgranular zone of the dentate gyrus.[9][10]
Structure

The dentate gyrus, like the hippocampus, consists of three distinct layers: an outer molecular layer, a middle granule cell layer, and an inner polymorphic layer.[11] (In the hippocampus the outer layer is the molecular layer, the middle layer is the pyramidal layer, and the inner layer the stratum oriens). The polymorphic layer is also the hilus of the dentate gyrus, (CA4, the junction of the hippocampus and dentate gyrus).[12][13]
The granule layer is between the overlying molecular layer and the underlying hilus (polymorphic layer).[10] The granule cells of the granule layer project their axons known as mossy fibers to make excitatory synapses on the dendrites of CA3 pyramidal neurons. The granule cells are tightly packed together in a laminated manner that dampens the excitability of neurons.[14]
Some of the basal dendrites of the granule cells curve up into the molecular layer. Most basal dendrites enter the hilus. These hilar dendrites are shorter and thinner, and have fewer side branches.[15]
A second excitatory cell type in the hilus is the mossy cell,[12] that projects its axons widely along the septotemporal axis, (running from the septal area to the temporal lobe) with the ipsilateral projection skipping the first 1–2 mm near the cell bodies,[16] an unusual configuration, hypothesized to prepare a set of cell assemblies in CA3 for a data retrieval role, by randomizing their cell distribution.[17]
Between the hilus and the granule cell layer is a region called the subgranular zone which is a site of adult neurogenesis.[10]
The anteromedial continuation of the dentate gyrus is called the tail of the dentate gyrus, or the band of Giacomini. Most of the dentate gyrus is not exposed on the surface of the brain but the band of Giacomini is visible, and makes an important landmark of the inferior surface of the uncus.[18]
Trisynaptic circuit
The trisynaptic circuit consists of excitatory cells (mostly stellate cells) in layer II of the entorhinal cortex, projecting to the granule cell layer of the dentate gyrus via the perforant path.[19][20] The dentate gyrus receives no direct inputs from other cortical structures.[21] The perforant path is divided into the medial and lateral perforant paths, generated, respectively, at the medial and lateral portions of the entorhinal cortex. The medial perforant path synapses onto the proximal dendritic area of the granule cells, whereas the lateral perforant path does so onto their distal dendrites. Most lateral views of the dentate gyrus may appear to suggest a structure consisting of just one entity, but medial movement may provide evidence of the ventral and dorsal parts of the dentate gyrus.[22] The axons of the granule cells called mossy fibres, make excitatory synaptic connections with the pyramidal cells of CA3 and CA1.[20]
Development
The granule cells in the dentate gyrus are distinguished by their late time of formation during brain development. In rats, approximately 85% of the granule cells are generated after birth.[23] In humans, it is estimated that granule cells begin to be generated during gestation weeks 10.5 to 11, and continue being generated during the second and third trimesters, after birth and all the way into adulthood.[24][25] The germinal sources of granule cells and their migration pathways[26] have been studied during rat brain development. The oldest granule cells are generated in a specific region of the hippocampal neuroepithelium and migrate into the primordial dentate gyrus around embryonic days (E) 17/18, and then settle as the outermost cells in the forming granular layer. Next, dentate precursor cells move out of this same area of the hippocampal neuroepithelium and, retaining their mitotic capacity, invade the hilus (core) of the forming dentate gyrus. This dispersed germinal matrix is the source of granule cells from that point on. The newly generated granule cells accumulate under the older cells that began to settle in the granular layer. As more granule cells are produced, the layer thickens and the cells are stacked up according to age - the oldest being the most superficial and the youngest being deeper.[27] The granule cell precursors remain in a subgranular zone that becomes progressively thinner as the dentate gyrus grows, but these precursor cells are retained in adult rats. These sparsely scattered cells constantly generate granule cell neurons,[28][29] which add to the total population. There are a variety of other differences in the rat, monkey and human dentate gyrus. The granule cells only have apical dendrites in the rat. But in the monkey and human, many granule cells also have basal dendrites.[1]
Function
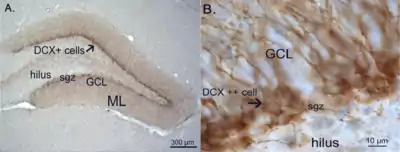
.jpg.webp)
The dentate gyrus is thought to contribute to the formation of memories, and to play a role in depression.
The role of the hippocampus in learning and memory has been studied for many decades particularly since the late 1950s, following the results of surgery, in an American male, to remove most of the hippocampus.[32] It remains unclear how the hippocampus enables new memory formation, but one process, called long term potentiation (LTP), occurs in this brain region.[33] LTP involves long-lasting strengthening of synaptic connections after repeated stimulation.[19] While the dentate gyrus shows LTP, it is also one of the few regions of the mammalian brain where adult neurogenesis (the formation of new neurons) takes place. Some studies hypothesize that new memories could preferentially use newly formed granule cells of the dentate gyrus, providing a potential mechanism for distinguishing multiple instances of similar events or multiple visits to the same location.[34] Correspondingly, it has been proposed that the immature, newborn granule cells are receptive to form new synaptic connections with the axons arriving from the layer II of the entorhinal cortex, this way a particular new constellation of events is remembered as an episodic memory by first associating the events in the young granule cells that have the appropriate, permissive age.[35] This concept is reinforced by the fact that increased neurogenesis is associated with improved spatial memory in rodents, as seen through performance in a maze.[36]
The dentate gyrus is known to serve as a pre-processing unit. While the CA3 subfield is involved in encoding, storage, and retrieval of memory, the dentate gyrus is important in pattern separation.[20] When information enters via the perforant path, the dentate gyrus separates very similar information into distinct and unique details.[37][38] This ensures that new memories are encoded separately without input from previously stored memories of similar feature,[10] and prepares the relevant data for storage in the CA3 region.[37] Pattern separation gives the ability to differentiate one memory from other stored memories.[39] Pattern separation begins in the dentate gyrus. Granule cells in the dentate gyrus process sensory information using competitive learning, and relay a preliminary representation to form place fields.[40] Place fields are extremely specific, as they are capable of remapping and adjusting firing rates in response to subtle sensory signal changes. This specificity is critical for pattern separation, as it distinguishes memories from one another.[39]
The dentate gyrus shows a specific form of neural plasticity resulting from the ongoing integration of newly formed excitatory granule cells.[10]
Clinical significance
Memory
One of the most prominent early cases of anterograde amnesia (inability to form new memories) linking the hippocampus to memory formation was the case of Henry Molaison (anonymously known as Patient H.M. until his death in 2008).[33] His epilepsy was treated with surgical removal of his hippocampi (left and right hemispheres each have their own hippocampus) as well as some surrounding tissue. This targeted brain tissue removal left Mr. Molaison with an inability to form new memories, and the hippocampus has been thought critical to memory formation since that time, though the processes involved are unclear.[33]
Stress and depression
The dentate gyrus may also have a functional role in stress and depression. For instance, in the rat, neurogenesis has been found to increase in response to chronic treatment with antidepressants.[41] The physiological effects of stress, often characterized by release of glucocorticoids such as cortisol, as well as activation of the sympathetic nervous system (a division of the autonomic nervous system), have been shown to inhibit the process of neurogenesis in primates.[42] Both endogenous and exogenous glucocorticoids are known to cause psychosis and depression,[43] implying that neurogenesis in the dentate gyrus may play an important role in modulating symptoms of stress and depression.[44]
Blood sugar
Studies by researchers at Columbia University Medical Center indicate that poor glucose control can lead to deleterious effects on the dentate gyrus, resulting in memory decline.[45]
Other
Some evidence seen in the mouse suggests that neurogenesis in the dentate gyrus increases in response to aerobic exercise.[46] Several experiments have shown neurogenesis (the development of nerve tissues) often increases in the dentate gyrus of adult rodents when they are exposed to an enriched environment.[47][48]
Mutations in a synapse-associated protein SAP97, a scaffold protein in the dentate gyrus might play a role in the susceptibility for schizophrenia.[49][50]
Spatial behavior
Studies have shown that after having about 90% of their dentate gyrus cells destroyed, rats had extreme difficulty in maneuvering through a maze they had previously negotiated. When being tested a number of times to see whether they could learn a maze, the results showed that the rats did not improve at all, indicating that their working memories were severely impaired. Rats had trouble with place strategies because they could not consolidate learned information about a maze into their working memory, and, thus, could not remember it when maneuvering through the same maze in a later trial. Every time a rat entered the maze, the rat behaved as if it was seeing the maze for the first time.[51]
References
- Amaral, David G.; Scharfman, Helen E.; Lavenex, Pierre (2007). "The dentate gyrus: Fundamental neuroanatomical organization (Dentate gyrus for dummies)". The Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications. Progress in Brain Research. Vol. 163. pp. 3–790. doi:10.1016/S0079-6123(07)63001-5. ISBN 9780444530158. PMC 2492885. PMID 17765709.
- Saab BJ, Georgiou J, Nath A, Lee FJ, Wang M, Michalon A, Liu F, Mansuy IM, Roder JC (2009). "NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory". Neuron. 63 (5): 643–56. doi:10.1016/j.neuron.2009.08.014. PMID 19755107. S2CID 5321020.
- Scharfman, Helen E., ed. (2011). The Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications. Elsevier. ISBN 978-0-08-055175-3.
- Cameron HA, McKay RD (July 2001). "Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus". J. Comp. Neurol. 435 (4): 406–17. doi:10.1002/cne.1040. PMID 11406822. S2CID 15254735.
- Ernst, A; Alkass, K; Bernard, S; Salehpour, M; Perl, S; Tisdale, J; Possnert, G; Druid, H; Frisén, J (27 February 2014). "Neurogenesis in the striatum of the adult human brain". Cell. 156 (5): 1072–83. doi:10.1016/j.cell.2014.01.044. PMID 24561062.
- Ponti G, Peretto P, Bonfanti L (2008). "Genesis of neuronal and glial progenitors in the cerebellar cortex of peripuberal and adult rabbits". PLOS ONE. 3 (6): e2366. Bibcode:2008PLoSO...3.2366P. doi:10.1371/journal.pone.0002366. PMC 2396292. PMID 18523645.
- Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, et al. (March 2018). "Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults". Nature. 555 (7696): 377–381. Bibcode:2018Natur.555..377S. doi:10.1038/nature25975. PMC 6179355. PMID 29513649.
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al. (April 2018). "Human Hippocampal Neurogenesis Persists throughout Aging". Cell Stem Cell. 22 (4): 589–599.e5. doi:10.1016/j.stem.2018.03.015. PMC 5957089. PMID 29625071.
- Abbott, Louise C.; Nigussie, Fikru (January 2020). "Adult neurogenesis in the mammalian dentate gyrus". Anatomia, Histologia, Embryologia. 49 (1): 3–16. doi:10.1111/ahe.12496. PMID 31568602.
- Tuncdemir, Sebnem Nur; Lacefield, Clay Orion; Hen, Rene (November 2019). "Contributions of adult neurogenesis to dentate gyrus network activity and computations". Behavioural Brain Research. 374: 112112. doi:10.1016/j.bbr.2019.112112. PMC 6724741. PMID 31377252.
- Treves, A.; Tashiro, A.; Witter, M.P.; Moser, E.I. (July 2008). "What is the mammalian dentate gyrus good for?". Neuroscience. 154 (4): 1155–1172. doi:10.1016/j.neuroscience.2008.04.073. PMID 18554812. S2CID 14710031.
- Scharfman, Helen E. (September 2016). "The enigmatic mossy cell of the dentate gyrus". Nature Reviews Neuroscience. 17 (9): 562–575. doi:10.1038/nrn.2016.87. PMC 5369357. PMID 27466143.
- Haines, D; Mihailoff, G (2018). Fundamental neuroscience for basic and clinical applications (Fifth ed.). p. 461. ISBN 9780323396325.
- Nadler, J. Victor (2003). "The recurrent mossy fiber pathway of the epileptic brain". Neurochemical Research. 28 (11): 1649–1658. doi:10.1023/a:1026004904199. PMID 14584819. S2CID 2566342.
- Seress, László; Mrzljak, Ladislav (March 1987). "Basal dendrites of granule cells are normal features of the fetal and adult dentate gyrus of both monkey and human hippocampal formations". Brain Research. 405 (1): 169–174. doi:10.1016/0006-8993(87)91003-1. PMID 3567591. S2CID 23358962.
- Amaral DG, Witter MP (1989). "The three-dimensional organization of the hippocampal formation: a review of anatomical data". Neuroscience. 31 (3): 571–591. doi:10.1016/0306-4522(89)90424-7. PMID 2687721. S2CID 28430607.
- Legéndy CR (2017). "On the 'data stirring' role of the dentate gyrus of the hippocampus". Reviews in the Neurosciences. 28 (6): 599–615. doi:10.1515/revneuro-2016-0080. PMID 28593904. S2CID 3716652.
- Elgendy, Azza. "Band of Giacomini | Radiology Reference Article | Radiopaedia.org". Radiopaedia. Retrieved 17 October 2019.
- Blumenfeld, Hal (2010). Neuroanatomy through clinical cases (2nd ed.). Sunderland, Mass.: Sinauer Associates. ISBN 978-0878936137.
- Senzai, Yuta (March 2019). "Function of local circuits in the hippocampal dentate gyrus-CA3 system". Neuroscience Research. 140: 43–52. doi:10.1016/j.neures.2018.11.003. PMID 30408501. S2CID 53220907.
- Nolte, John (2002). The Human Brain: An Introduction to Its Functional Neuroanatomy (fifth ed.). pp. 570–573.
- Rachel A. Dalley; Lydia L. Ng; Angela L. Guillozet-Bongaarts (2008). "Dentate Gyrus". Nature Precedings. doi:10.1038/npre.2008.2095.1.
- Bayer SA, Altman J (November 1974). "Hippocampal development in the rat: cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation". J. Comp. Neurol. 158 (1): 55–79. doi:10.1002/cne.901580105. PMID 4430737. S2CID 17968282.
- Bayer SA, Altman J (2008). The Human Brain During The Early First Trimester. Vol. 5 Atlas of Human Central Nervous System Development. Appendix, p. 497.
- Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. (November 1998). "Neurogenesis in the adult human hippocampus". Nat. Med. 4 (11): 1313–7. doi:10.1038/3305. PMID 9809557.
- Altman J, Bayer SA (November 1990). "Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods". J. Comp. Neurol. 301 (3): 365–81. doi:10.1002/cne.903010304. PMID 2262596. S2CID 7425653.
- Angevine JB (October 1965). "Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse". Exp Neurol Suppl (Suppl 2): Suppl 2:1–70. PMID 5838955.
- Bayer SA, Yackel JW, Puri PS (May 1982). "Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life". Science. 216 (4548): 890–2. Bibcode:1982Sci...216..890B. doi:10.1126/science.7079742. PMID 7079742.
- Bayer SA (1982). "Changes in the total number of dentate granule cells in juvenile and adult rats: a correlated volumetric and 3H-thymidine autoradiographic study". Exp Brain Res. 46 (3): 315–23. doi:10.1007/bf00238626. PMID 7095040. S2CID 18663323.
- Oomen, Charlotte A.; Girardi, Carlos E. N.; Cahyadi, Rudy; Verbeek, Eva C.; Krugers, Harm; Joëls, Marian; Lucassen, Paul J. (29 January 2009). "Opposite Effects of Early Maternal Deprivation on Neurogenesis in Male versus Female Rats". PLOS ONE. 4 (1): e3675. Bibcode:2009PLoSO...4.3675O. doi:10.1371/journal.pone.0003675. PMC 2629844. PMID 19180242.
- Faiz M, Acarin L, Castellano B, Gonzalez B (2005). "Proliferation dynamics of germinative zone cells in the intact and excitotoxically lesioned postnatal rat brain". BMC Neurosci. 6 (1): 26. doi:10.1186/1471-2202-6-26. PMC 1087489. PMID 15826306.
- Benedict Carey (4 December 2008). "H. M., an Unforgettable Amnesiac, Dies at 82". The New York Times. Retrieved 5 December 2008.
In 1953, he underwent an experimental brain operation in Hartford to correct a seizure disorder, only to emerge from it fundamentally and irreparably changed. He developed a syndrome neurologists call profound amnesia. He had lost the ability to form new declarative memories.
- Kandel ER, Schwartz J, Jessell T, Siegelbaum S, Hudspeth AJ (2013). Principles of neural science (5th ed.). McGraw Hill Professional. ISBN 978-0-07-139011-8.
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, et al. (March 2012). "Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion". Cell. 149 (1): 188–201. doi:10.1016/j.cell.2012.01.046. PMC 3319279. PMID 22365813.
- Kovács KA (September 2020). "Episodic Memories: How do the Hippocampus and the Entorhinal Ring Attractors Cooperate to Create Them?". Frontiers in Systems Neuroscience. 14: 68. doi:10.3389/fnsys.2020.559186. PMC 7511719. PMID 33013334.
- Bliss RM (August 2007). ""Food and the Aging Mind". First in a Series: Nutrition and Brain Function". USDA. USDA.gov. Retrieved 27 February 2010.
- Jonas, Peter; Lisman, John (10 September 2014). "Structure, function, and plasticity of hippocampal dentate gyrus microcircuits". Frontiers in Neural Circuits. 8: 107. doi:10.3389/fncir.2014.00107. PMC 4159971. PMID 25309334.
- Lamothe-Molina, Paul J.; Franzelin, Andreas; Auksutat, Lea; Laprell, Laura; Alhbeck, Joachim; Kneussel, Matthias; Engel, Andreas K.; Morellini, Fabio; Oertner, Thomas G. (31 August 2020). "cFos ensembles in the dentate gyrus rapidly segregate over time and do not form a stable map of space" (PDF). doi:10.1101/2020.08.29.273391. S2CID 221510080.
{{cite journal}}: Cite journal requires|journal=(help) - Moser, Edvard I.; Kropff, Emilio; Moser, May-Britt (1 July 2008). "Place Cells, Grid Cells, and the Brain's Spatial Representation System". Annual Review of Neuroscience. 31 (1): 69–89. doi:10.1146/annurev.neuro.31.061307.090723. PMID 18284371.
- Rolls, Edmund T. (2013). "The mechanisms for pattern completion and pattern separation in the hippocampus". Frontiers in Systems Neuroscience. 7: 74. doi:10.3389/fnsys.2013.00074. PMC 3812781. PMID 24198767.
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS (December 2000). "Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus". J. Neurosci. 20 (24): 9104–10. doi:10.1523/JNEUROSCI.20-24-09104.2000. PMC 6773038. PMID 11124987.
- Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E (March 1998). "Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress". Proc. Natl. Acad. Sci. U.S.A. 95 (6): 3168–71. Bibcode:1998PNAS...95.3168G. doi:10.1073/pnas.95.6.3168. PMC 19713. PMID 9501234.
- Jacobs BL, van Praag H, Gage FH (May 2000). "Adult brain neurogenesis and psychiatry: a novel theory of depression". Mol. Psychiatry. 5 (3): 262–9. doi:10.1038/sj.mp.4000712. PMID 10889528.
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C (December 2011). "Antidepressants recruit new neurons to improve stress response regulation". Mol. Psychiatry. 16 (12): 1177–88. doi:10.1038/mp.2011.48. PMC 3223314. PMID 21537331.
- "Blood Sugar Control Linked to Memory Decline, Study Says". The New York Times. 1 January 2009. Retrieved 13 March 2011.
- Praag, H (1999). "Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus". Nature Neuroscience. 2 (3): 266–270. doi:10.1038/6368. PMID 10195220. S2CID 7170664.
- Kempermann G, Kuhn HG, Gage FH (April 1997). "More hippocampal neurons in adult mice living in an enriched environment". Nature. 386 (6624): 493–5. Bibcode:1997Natur.386..493K. doi:10.1038/386493a0. PMID 9087407. S2CID 4281128.
- Eadie BD, Redila VA, Christie BR (May 2005). "Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density". J. Comp. Neurol. 486 (1): 39–47. doi:10.1002/cne.20493. hdl:2429/15467. PMID 15834963. S2CID 8386870.
- Boccitto M, Doshi S, Newton IP, Nathke I, Neve R, Dong F, Mao Y, Zhai J, Zhang L, Kalb R (June 2016). "Opposing actions of the synapse-associated protein of 97-kDa molecular weight (SAP97) and Disrupted in Schizophrenia 1 (DISC1) on Wnt/β-catenin signaling". Neuroscience. 326: 22–30. doi:10.1016/j.neuroscience.2016.03.048. PMC 4853273. PMID 27026592.
- Kay, Yuni; Tsan, Linda; Davis, Elizabeth A.; Tian, Chen; Décarie-Spain, Léa; Sadybekov, Anastasiia; Pushkin, Anna N.; Katritch, Vsevolod; Kanoski, Scott E.; Herring, Bruce E. (December 2022). "Schizophrenia-associated SAP97 mutations increase glutamatergic synapse strength in the dentate gyrus and impair contextual episodic memory in rats". Nature Communications. 13 (1): 798. doi:10.1038/s41467-022-28430-5. PMC 8831576. PMID 35145085. S2CID 246704130.
- Xavier GF, Costa VC (August 2009). "Dentate gyrus and spatial behaviour". Prog. Neuropsychopharmacol. Biol. Psychiatry. 33 (5): 762–73. doi:10.1016/j.pnpbp.2009.03.036. PMID 19375476. S2CID 1115081.
External links
- Slide at psycheducation.org
- Stained brain slice images which include the "Dentate gyrus" at the BrainMaps project
- McHugh TJ (2007). "Dentate Gyrus NMDA Receptors Mediate Rapid Pattern Separation in the Hippocampal Network". Science. 317 (5834): 94–99. Bibcode:2007Sci...317...94M. doi:10.1126/science.1140263. PMID 17556551. S2CID 18548. - The source of déjà vu
- NIF Search - Dentate Gyrus via the Neuroscience Information Framework
- See Altman and Bayer's work on dentate gyrus development and adult neurogenesis
- Amaral, David G.; Scharfman, Helen E.; Lavenex, Pierre (2007). "The dentate gyrus: Fundamental neuroanatomical organization (Dentate gyrus for dummies)". The Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications. Progress in Brain Research. Vol. 163. pp. 3–790. doi:10.1016/S0079-6123(07)63001-5. ISBN 9780444530158. PMC 2492885. PMID 17765709.