Developmental toxicity
Developmental toxicity is any structural or functional alteration, reversible or irreversible, which interferes with homeostasis, normal growth, differentiation, development or behavior, and is caused by environmental insult (including drugs, lifestyle factors such as alcohol, diet, and environmental toxic chemicals or physical factors). It is the study of adverse effects on the development of the organism resulting from exposure to toxic agents before conception (either parent), during prenatal development, or post-natally until puberty.[1] The substance that causes developmental toxicity from embryonic stage to birth is called teratogens. The effect of the developmental toxicants depends on the type of substance, dose and duration and time of exposure.
Certain pathogens are also included since the toxins they secrete are known to cause adverse effects on the development of the organism when the mother or fetus is infected. Developmental toxicology is a science studying adverse developmental outcomes. This term has widely replaced the early term for the study of primarily structural congenital abnormalities, teratology, to enable inclusion of a more diverse spectrum of congenital disorders. Typical factors causing developmental toxicity are radiation, infections (e.g. rubella), maternal metabolic imbalances (e.g. alcoholism, diabetes, folic acid deficiency), drugs (e.g. anticancer drugs, tetracyclines, many hormones, thalidomide), and environmental chemicals (e.g. mercury, lead, dioxins, PBDEs, HBCD, tobacco smoke). The first-trimester exposure is considered the most potential for developmental toxicity.
Once fertilization has taken place, the toxicants in the environment can pass through the mother to the developing embryo or fetus across the placental barrier. The fetus is at greatest risk during the first 14th to 60th day of the pregnancy when the major organs are being formed. However, depending on the type of toxicant and amount of exposure, a fetus can be exposed to toxicants at any time during pregnancy. For example, exposure to a particular toxicant at one time in the pregnancy may result in organ damage and at another time in the pregnancy could cause death of the fetus and miscarriage. There are a number of chemicals, biological agents (such as bacteria and viruses), and physical agents (such as radiation) used in a variety of workplaces that are known to cause developmental disorders.[2] Developmental disorders can include a wide range of physical abnormalities, such as bone or organ deformities, or behavioral and learning problems, such as an intellectual disability.[3] Exposures to some chemicals during pregnancy can lead to the development of cancer later in the life of the child and are called transgenerational carcinogens. Exposure to toxicants during the second and third trimesters of a pregnancy can lead to slow fetal grown and result in low birth weight.
History
Researchers have been able to ascertain toxicity associated with abnormal development with new breakthrough in developmental biology. Recognition of the developmental toxic effects of various molecules is recent development.
Terato means monster in Greek. Until the 18th century, the preformism theory was accepted by which abnormal growth was considered as deformations. The 19th century saw developmental in descriptive embryology where abnormalities were now considered as malformations or errors during a developmental process giving rise to the concept of teratogenesis. By the 20th century, the concept of epigenesis the interaction between a genetic program and environment was established and in the second half of the 20th century researchers had evidence that environmental factors can cause malformations and even trans-generational effects.[4]
Maternal irradiation and congenital malformations
One of the first environmentally induced congenital malformations in humans were recognized as a result of maternal irradiation. Hiroshima (1953) and Nagasaki (1955) had ascertained this fact for the first time based on the records of births occurring before May 31, 1946, but after the atomic bombing (August 6, 1945, in Hiroshima; August 9, 1945, in Nagasaki). A 20% increase in microcephaly frequency was seen in children with in-utero radiation exposure during the first trimester of the pregnancy (Miller 1956, 1968). Sensitivity to these radiations was seen to be predominantly high during the 7–15th week of gestation.
Two pertinent points were observed during this study:
- The severity and frequency of the congenital abnormalities seen increased with dose of radiation which depended on the closeness to the source or explosion.
- It was determined that there were critical periods of pregnancy when these exposures had the maximum effect on the fetal development.
_PHIL_4284_lores.jpg.webp)
Congenital rubella syndrome (CRS)
Rubella was the first recognized human epidemic of malformations. Following a widespread epidemic of rubella infection in 1940, Norman Gregg, an Australian ophthalmologist, reported in 1941 the occurrence of congenital cataracts among 78 infants born following maternal rubella infection in early pregnancy. This indicated that the virus had to cross the placental barrier to reach the fetus and cause malformations. The time of exposure to the virus also had a direct impact on the incidence of congenital malformations with exposure during week 4, 5–8 and 9–12 weeks of pregnancy caused 61%, 26% and 8% of congenital malformations. This was the first published recognition of congenital rubella syndrome (CRS). The progeny had congenital eye, heart and ear defects as well as intellectual disability.[5]
Thalidomide Tragedy (1950)
Thalidomide was extensively used for the treatment of nausea in pregnant women in the late 1950s and early 1960s until it became apparent in the 1960s that it resulted in severe birth defects. Fetus that were exposed to thalidomide while in the womb experienced limb malformation by which the limb were not developed or appeared as stumps. Other effects also seen with thalidomide exposure included deformed eyes and hearts, deformed alimentary and urinary tracts, blindness and deafness.[6] The thalidomide tragedy marked a turning point in toxicity testing, as it prompted United States and international regulatory agencies to develop systematic toxicity testing protocol. The effects of thalidomide led to important discoveries in the biochemical pathways of limb development.[7]
Testing and risk assessment
Testing for developmental toxicant is done in different stages:
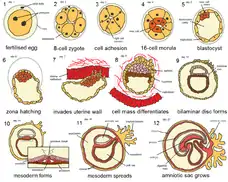
- Fertilization to implantation - Fertilization followed by increase in cell number, cleavage and cavitation to form the blastocyst which gets implanted. Toxicant exposure at this stage usually prevents implantation and results in death. e.g. DDT, nicotine
- Implantation to gastrulation - The three germ layers are formed and the cells start migrating out to initiate organogenesis. This is most sensitive stage for alcohol toxicity.
- Organogenesis - It is the formation of limbs, organs, nervous system, urinary and genital systems by the process of cell differentiation, migration and cell interactions from the 3rd to 8th week of human gestation. e.g. DES
- Morphogenesis - Includes the stages of growth and physiological maturation from week 8 until birth. Teratogenic effects results in deformations and rather than malformations in the fetus.
- Post Natal to puberty - Environmental toxicant exposures.
Because of the complexity of the embryo-fetal development, including the maternal-fetal interactions during gestation, it is important to understand the mechanism of toxicity and test the toxic effect in more than two species before confirming the substance to be a developmental toxicant. Embryo have different critical periods for the organ formation from day 15 to day 60 and hence the susceptibility to toxicant injury is directly related to the period of development.
Toxicity effects
Developmental toxicity is the alterations of the developmental processes (organogenesis, morphogenesis) rather than functional alterations of already developed organs. The effects of the toxicants depends on the dose, threshold and duration. The effects of toxicity are:
- Minor structural deformities - e.g. Anticonvulsant drugs, Warfarin, Retinoic Acid derivatives
- Major structural deformities - e.g. DES (diethylstilbestrol), cigarette smoking
- Growth Retardation - e.g. Alcohol, Polychlorinated Biphenyls
- Functional alterations - e.g. Retinoic Acid derivatives, Polychlorinated Biphenyls, Phenobarbitol, Lead
- Death- e.g. Rubella, ACE inhibitors
Effects on neurulation
Neurulation is one of the most important stages in the development of vertebrates. It is the process of formation of a flat neural plate which then convolutes to form the hollow neural tube.[8] It is considered to be one of the main targets of developmental toxicity and defects in neurulation is a common consequence of toxicant exposure and results in large proportion of human defects.[9]
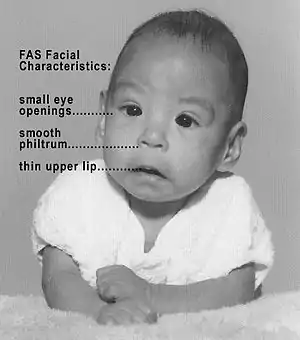
Fetal alcohol syndrome (FAS)
Fetal alcohol spectrum disorders (FASD) is a term that constitutes the set of conditions that can occur in a person whose mother drank alcohol during the course of pregnancy. These effects can include physical and cognitive problems. FASD patient usually has a combination of these problems.[10] Extent of effect depends on exposure frequency, dose and rate of ethanol elimination from amniotic fluid. FAS disrupts normal development of the fetus, which may cause certain developmental stages to be delayed, skipped, or immaturely developed.[11] Since alcohol elimination is slow in a fetus than in an adult and the fact that they do not have a developed liver to metabolize the alcohol, alcohol levels tend to remain high and stay in the fetus longer. Birth defects associated with prenatal exposure to alcohol can occur in the first three to eight weeks of pregnancy before a woman even knows that she is pregnant.[12]
_cervix_(5).jpg.webp)
DES (diethylstilbestrol)
DES (diethylstilbestrol) is a drug that mimics estrogen, a female hormone. From 1938 until 1971, doctors prescribed this drug to help some pregnant women who had had miscarriages or premature deliveries on the theory that miscarriages and premature births occurred because some pregnant women did not produce enough estrogen naturally to sustain the pregnancy for full term. An estimated 5–10 million pregnant women and the children born during this period were exposed to DES. Currently, DES is known to increase the risk of breast cancer, and cause a variety of birth-related adverse outcomes exposed female offsprings such as spontaneous abortion, second-trimester pregnancy loss, preterm delivery, stillbirth, neonatal death, sub/infertility and cancer of reproductive tissues. DES is an important developmental toxicant which links the fetal basis of adult disease.[13]
Methylmercury
Methylmercury and inorganic mercury is excreted in human breast milk and infants are particularly susceptible to toxicity due to this compound.[14] The fetus and infant are especially vulnerable to mercury exposures with special interest in the development of the CNS since it can easily cross across the placental barrier, accumulate within the placenta and fetus as the fetus cannot eliminate mercury and have a negative effect on the fetus even if the mother does not show symptoms.[15] Mercury causes damage to the nervous system resulting from prenatal or early postnatal exposure and is very likely to be permanent.[16]
Chlorpyrifos
It is an organophosphate insecticide that acts on the nervous system of insects by inhibiting acetylcholinesterase but are moderately toxic to humans. But it is known have developmental effects appear in fetuses and children even at very small doses. It has been shown to cause abnormal reflexes in neonates, poorer mental development in 2 and 3 year olds, poorer verbal IQ in 3+1⁄2 and 5 year old and pervasive developmental disorder in 2, 3 and 3+1⁄2 year olds.[17]
Environmental endocrine disruptors
Endocrine disruptors are molecules that alter the structure or function of the endocrine system like DDT, BPA etc. Prenatal BPA exposure is associated with aggression and neurobehaviour changes.[18]
Epigenetics
Most toxicants are known to affect only a fraction of exposed population. This is due to the differences in the genetic makeup of the organisms which affects toxicant metabolism and clearance from the body. Effect of developmental toxicants depends on the genetic makeup of the mother and fetus.[19]
Major developmental toxicants
Some of the known developmental toxicants can be grouped under the following categories:
Reproductive toxins:
- Aminopterin
- Methotrexate
- Androgen
- ACE inhibitor
- Antituberculous drug
- Caffeine
- Cocaine
- Coumarin
- Diethylstilbestrol
- Ethanol
- Insulin shock therapy
- Isotretinoin
- Streptomycin
- Thalidomide
- Trimethoprim
- Vitamin A
- Vitamin D
- Warfarin
Anti-convulsants:
Chemicals:
- Lead
- Gasoline
- Methylmercury
- Polychlorinated biphenyl
- Toluene toxicity
Biological agents:
- Cytomegalovirus
- Rubella
- Herpes simplex virus
- HIV
- Syphilis
- Toxoplasmosis
- Varicella zoster virus
- Venezuelan equine encephalitis virus
Lifestyle:
Maternal metabolic imbalances:
References
- Klaassen, Curtis; III, John B. Watkins (2003-06-26). Casarett & Doull's Essentials of Toxicology. McGraw-Hill Companies, Incorporated. ISBN 9780071389143.
- Sharon L.B.S., Drozdowsky. "Workplace Hazards to Reproduction and Development: A Resource for Workers, Employers, Health Care Providers, and Health & Safety Personnel" (PDF). Safety and Health Assessment and Research for Prevention (SHARP) Washington State Department of Labor and Industries. Retrieved April 14, 2016.
- Julvez, Jordi; Grandjean, Philippe (2009-10-01). "Neurodevelopmental toxicity risks due to occupational exposure to industrial chemicals during pregnancy". Industrial Health. 47 (5): 459–468. doi:10.2486/indhealth.47.459. ISSN 1880-8026. PMID 19834254.
- Sander, Klaus (1997). Landmarks in Developmental Biology 1883–1924 - Springer. doi:10.1007/978-3-642-60492-8. ISBN 978-3-642-64428-3. S2CID 11288064.
- "Pinkbook | Rubella | Epidemiology of Vaccine Preventable Diseases | CDC". www.cdc.gov. Retrieved 2016-04-13.
- "JSONpedia - Thalidomide". jsonpedia.org. Retrieved 2016-04-14.
- Kim, James H.; Scialli, Anthony R. (2011-07-01). "Thalidomide: the tragedy of birth defects and the effective treatment of disease". Toxicological Sciences. 122 (1): 1–6. doi:10.1093/toxsci/kfr088. ISSN 1096-0929. PMID 21507989.
- Gilbert, Scott (2014). Developmental biology. Sunderland, MA: Sinauer Associates, Inc.
- CaroleAKimmel (1994-06-30). Developmental Toxicology. CRC Press. ISBN 9780781701372.
- "Fetal Alcohol Spectrum Disorders: MedlinePlus". www.nlm.nih.gov. Retrieved 2016-04-14.
- B., McCreight (1997). Recognizing and Managing Children with Fetal Alcohol Syndrome/Fetal Alcohol Effects: A Guidebook.
- "Home | FASD | NCBDDD | CDC". www.cdc.gov. Retrieved 2016-04-13.
- Reed, Casey E.; Fenton, Suzanne E. (2013-06-01). "Exposure to Diethylstilbestrol during Sensitive Life Stages: A legacy of heritable health effects". Birth Defects Research Part C: Embryo Today: Reviews. 99 (2): 134–46. doi:10.1002/bdrc.21035. ISSN 1542-975X. PMC 3817964. PMID 23897597.
- Yang, J.; Jiang, Z.; Wang, Y.; Qureshi, I. A.; Wu, X. D. (1997-04-01). "Maternal-fetal transfer of metallic mercury via the placenta and milk". Annals of Clinical and Laboratory Science. 27 (2): 135–141. ISSN 0091-7370. PMID 9098513.
- Harada, M. (1995-01-01). "Minamata disease: methylmercury poisoning in Japan caused by environmental pollution". Critical Reviews in Toxicology. 25 (1): 1–24. doi:10.3109/10408449509089885. ISSN 1040-8444. PMID 7734058.
- Rice, D.; Barone, S. (2000-06-01). "Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models". Environmental Health Perspectives. 108 Suppl 3: 511–533. doi:10.2307/3454543. ISSN 0091-6765. JSTOR 3454543. PMC 1637807. PMID 10852851.
- "EPA Archives". archive.epa.gov. Retrieved 2016-04-14.
- Braun, Joe M.; Yolton, Kimberly; Dietrich, Kim N.; Hornung, Richard; Ye, Xiaoyun; Calafat, Antonia M.; Lanphear, Bruce P. (2009-12-01). "Prenatal Bisphenol A Exposure and Early Childhood Behavior". Environmental Health Perspectives. 117 (12): 1945–1952. doi:10.1289/ehp.0900979. ISSN 0091-6765. PMC 2799471. PMID 20049216.
- Watson, Rebecca E.; Goodman, Jay I. (2002-05-01). "Epigenetics and DNA Methylation Come of Age in Toxicology". Toxicological Sciences. 67 (1): 11–16. doi:10.1093/toxsci/67.1.11. ISSN 1096-6080. PMID 11961211.
Sources
- J.M. Rogers; R.J. Kavlock (2001). "Developmental toxicology". In C.D. Klaassen (ed.). Casarett & Doull's Toxicology (6th ed.). New York: McGraw-Hill. pp. 351–386. ISBN 978-0-07-134721-1.