Developmental biology
Developmental biology is the study of the process by which animals and plants grow and develop. Developmental biology also encompasses the biology of regeneration, asexual reproduction, metamorphosis, and the growth and differentiation of stem cells in the adult organism.
| Part of a series on |
| Biology |
|---|
|
|
Perspectives
The main processes involved in the embryonic development of animals are: tissue patterning (via regional specification and patterned cell differentiation); tissue growth; and tissue morphogenesis.
- Regional specification refers to the processes that create the spatial patterns in a ball or sheet of initially similar cells. This generally involves the action of cytoplasmic determinants, located within parts of the fertilized egg, and of inductive signals emitted from signaling centers in the embryo. The early stages of regional specification do not generate functional differentiated cells, but cell populations committed to developing to a specific region or part of the organism. These are defined by the expression of specific combinations of transcription factors.
- Cell differentiation relates specifically to the formation of functional cell types such as nerve, muscle, secretory epithelia, etc. Differentiated cells contain large amounts of specific proteins associated with cell function.
- Morphogenesis relates to the formation of a three-dimensional shape. It mainly involves the orchestrated movements of cell sheets and of individual cells. Morphogenesis is important for creating the three germ layers of the early embryo (ectoderm, mesoderm, and endoderm) and for building up complex structures during organ development.
- Tissue growth involves both an overall increase in tissue size, and also the differential growth of parts (allometry) which contributes to morphogenesis. Growth mostly occurs through cell proliferation but also through changes in cell size or the deposition of extracellular materials.
The development of plants involves similar processes to that of animals. However, plant cells are mostly immotile so morphogenesis is achieved by differential growth, without cell movements. Also, the inductive signals and the genes involved are different from those that control animal development.
Developmental processes
Cell differentiation
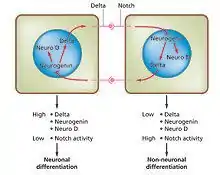
Cell differentiation is the process whereby different functional cell types arise in development. For example, neurons, muscle fibers and hepatocytes (liver cells) are well known types of differentiated cells. Differentiated cells usually produce large amounts of a few proteins that are required for their specific function and this gives them the characteristic appearance that enables them to be recognized under the light microscope. The genes encoding these proteins are highly active. Typically their chromatin structure is very open, allowing access for the transcription enzymes, and specific transcription factors bind to regulatory sequences in the DNA in order to activate gene expression.[1][2] For example, NeuroD is a key transcription factor for neuronal differentiation, myogenin for muscle differentiation, and HNF4 for hepatocyte differentiation. Cell differentiation is usually the final stage of development, preceded by several states of commitment which are not visibly differentiated. A single tissue, formed from a single type of progenitor cell or stem cell, often consists of several differentiated cell types. Control of their formation involves a process of lateral inhibition,[3] based on the properties of the Notch signaling pathway.[4] For example, in the neural plate of the embryo this system operates to generate a population of neuronal precursor cells in which NeuroD is highly expressed.
Regeneration
Regeneration indicates the ability to regrow a missing part.[5] This is very prevalent amongst plants, which show continuous growth, and also among colonial animals such as hydroids and ascidians. But most interest by developmental biologists has been shown in the regeneration of parts in free living animals. In particular four models have been the subject of much investigation. Two of these have the ability to regenerate whole bodies: Hydra, which can regenerate any part of the polyp from a small fragment,[6] and planarian worms, which can usually regenerate both heads and tails.[7] Both of these examples have continuous cell turnover fed by stem cells and, at least in planaria, at least some of the stem cells have been shown to be pluripotent.[8] The other two models show only distal regeneration of appendages. These are the insect appendages, usually the legs of hemimetabolous insects such as the cricket,[9] and the limbs of urodele amphibians.[10] Considerable information is now available about amphibian limb regeneration and it is known that each cell type regenerates itself, except for connective tissues where there is considerable interconversion between cartilage, dermis and tendons. In terms of the pattern of structures, this is controlled by a re-activation of signals active in the embryo. There is still debate about the old question of whether regeneration is a "pristine" or an "adaptive" property.[11] If the former is the case, with improved knowledge, we might expect to be able to improve regenerative ability in humans. If the latter, then each instance of regeneration is presumed to have arisen by natural selection in circumstances particular to the species, so no general rules would be expected.
Embryonic development of animals
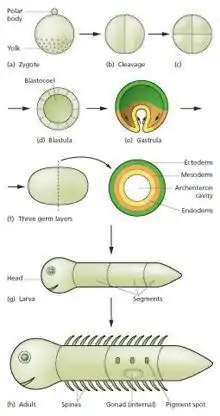
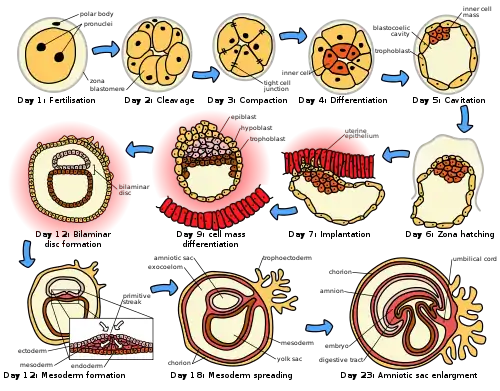
The sperm and egg fuse in the process of fertilization to form a fertilized egg, or zygote.[12] This undergoes a period of divisions to form a ball or sheet of similar cells called a blastula or blastoderm. These cell divisions are usually rapid with no growth so the daughter cells are half the size of the mother cell and the whole embryo stays about the same size. They are called cleavage divisions.
Mouse epiblast primordial germ cells (see Figure: “The initial stages of human embryogenesis”) undergo extensive epigenetic reprogramming.[13] This process involves genome-wide DNA demethylation, chromatin reorganization and epigenetic imprint erasure leading to totipotency.[13] DNA demethylation is carried out by a process that utilizes the DNA base excision repair pathway.[14]
Morphogenetic movements convert the cell mass into a three layered structure consisting of multicellular sheets called ectoderm, mesoderm and endoderm. These sheets are known as germ layers. This is the process of gastrulation. During cleavage and gastrulation the first regional specification events occur. In addition to the formation of the three germ layers themselves, these often generate extraembryonic structures, such as the mammalian placenta, needed for support and nutrition of the embryo,[15] and also establish differences of commitment along the anteroposterior axis (head, trunk and tail).[16]
Regional specification is initiated by the presence of cytoplasmic determinants in one part of the zygote. The cells that contain the determinant become a signaling center and emit an inducing factor. Because the inducing factor is produced in one place, diffuses away, and decays, it forms a concentration gradient, high near the source cells and low further away.[17][18] The remaining cells of the embryo, which do not contain the determinant, are competent to respond to different concentrations by upregulating specific developmental control genes. This results in a series of zones becoming set up, arranged at progressively greater distance from the signaling center. In each zone a different combination of developmental control genes is upregulated.[19] These genes encode transcription factors which upregulate new combinations of gene activity in each region. Among other functions, these transcription factors control expression of genes conferring specific adhesive and motility properties on the cells in which they are active. Because of these different morphogenetic properties, the cells of each germ layer move to form sheets such that the ectoderm ends up on the outside, mesoderm in the middle, and endoderm on the inside.[20][21] Morphogenetic movements not only change the shape and structure of the embryo, but by bringing cell sheets into new spatial relationships they also make possible new phases of signaling and response between them.
Growth in embryos is mostly autonomous.[22] For each territory of cells the growth rate is controlled by the combination of genes that are active. Free-living embryos do not grow in mass as they have no external food supply. But embryos fed by a placenta or extraembryonic yolk supply can grow very fast, and changes to relative growth rate between parts in these organisms help to produce the final overall anatomy.
The whole process needs to be coordinated in time and how this is controlled is not understood. There may be a master clock able to communicate with all parts of the embryo that controls the course of events, or timing may depend simply on local causal sequences of events.[23]
Metamorphosis
Developmental processes are very evident during the process of metamorphosis. This occurs in various types of animal. Well-known examples are seen in frogs, which usually hatch as a tadpole and metamorphoses to an adult frog, and certain insects which hatch as a larva and then become remodeled to the adult form during a pupal stage.
All the developmental processes listed above occur during metamorphosis. Examples that have been especially well studied include tail loss and other changes in the tadpole of the frog Xenopus,[24][25] and the biology of the imaginal discs, which generate the adult body parts of the fly Drosophila melanogaster.[26][27]
Plant development
Plant development is the process by which structures originate and mature as a plant grows. It is studied in plant anatomy and plant physiology as well as plant morphology.
Plants constantly produce new tissues and structures throughout their life from meristems[28] located at the tips of organs, or between mature tissues. Thus, a living plant always has embryonic tissues. By contrast, an animal embryo will very early produce all of the body parts that it will ever have in its life. When the animal is born (or hatches from its egg), it has all its body parts and from that point will only grow larger and more mature.
The properties of organization seen in a plant are emergent properties which are more than the sum of the individual parts. "The assembly of these tissues and functions into an integrated multicellular organism yields not only the characteristics of the separate parts and processes but also quite a new set of characteristics which would not have been predictable on the basis of examination of the separate parts."[29]
Growth
A vascular plant begins from a single celled zygote, formed by fertilisation of an egg cell by a sperm cell. From that point, it begins to divide to form a plant embryo through the process of embryogenesis. As this happens, the resulting cells will organize so that one end becomes the first root, while the other end forms the tip of the shoot. In seed plants, the embryo will develop one or more "seed leaves" (cotyledons). By the end of embryogenesis, the young plant will have all the parts necessary to begin its life.
Once the embryo germinates from its seed or parent plant, it begins to produce additional organs (leaves, stems, and roots) through the process of organogenesis. New roots grow from root meristems located at the tip of the root, and new stems and leaves grow from shoot meristems located at the tip of the shoot.[30] Branching occurs when small clumps of cells left behind by the meristem, and which have not yet undergone cellular differentiation to form a specialized tissue, begin to grow as the tip of a new root or shoot. Growth from any such meristem at the tip of a root or shoot is termed primary growth and results in the lengthening of that root or shoot. Secondary growth results in widening of a root or shoot from divisions of cells in a cambium.[31]
In addition to growth by cell division, a plant may grow through cell elongation.[32] This occurs when individual cells or groups of cells grow longer. Not all plant cells will grow to the same length. When cells on one side of a stem grow longer and faster than cells on the other side, the stem will bend to the side of the slower growing cells as a result. This directional growth can occur via a plant's response to a particular stimulus, such as light (phototropism), gravity (gravitropism), water, (hydrotropism), and physical contact (thigmotropism).
Plant growth and development are mediated by specific plant hormones and plant growth regulators (PGRs) (Ross et al. 1983).[33] Endogenous hormone levels are influenced by plant age, cold hardiness, dormancy, and other metabolic conditions; photoperiod, drought, temperature, and other external environmental conditions; and exogenous sources of PGRs, e.g., externally applied and of rhizospheric origin.
Morphological variation
Plants exhibit natural variation in their form and structure. While all organisms vary from individual to individual, plants exhibit an additional type of variation. Within a single individual, parts are repeated which may differ in form and structure from other similar parts. This variation is most easily seen in the leaves of a plant, though other organs such as stems and flowers may show similar variation. There are three primary causes of this variation: positional effects, environmental effects, and juvenility.
Evolution of plant morphology
Transcription factors and transcriptional regulatory networks play key roles in plant morphogenesis and their evolution. During plant landing, many novel transcription factor families emerged and are preferentially wired into the networks of multicellular development, reproduction, and organ development, contributing to more complex morphogenesis of land plants.[34]
Most land plants share a common ancestor, multicellular algae. An example of the evolution of plant morphology is seen in charophytes. Studies have shown that charophytes have traits that are homologous to land plants. There are two main theories of the evolution of plant morphology, these theories are the homologous theory and the antithetic theory. The commonly accepted theory for the evolution of plant morphology is the antithetic theory. The antithetic theory states that the multiple mitotic divisions that take place before meiosis, cause the development of the sporophyte. Then the sporophyte will development as an independent organism.[35]
Developmental model organisms
Much of developmental biology research in recent decades has focused on the use of a small number of model organisms. It has turned out that there is much conservation of developmental mechanisms across the animal kingdom. In early development different vertebrate species all use essentially the same inductive signals and the same genes encoding regional identity. Even invertebrates use a similar repertoire of signals and genes although the body parts formed are significantly different. Model organisms each have some particular experimental advantages which have enabled them to become popular among researchers. In one sense they are "models" for the whole animal kingdom, and in another sense they are "models" for human development, which is difficult to study directly for both ethical and practical reasons. Model organisms have been most useful for elucidating the broad nature of developmental mechanisms. The more detail is sought, the more they differ from each other and from humans.
Plants
- Thale cress (Arabidopsis thaliana)[36]
Vertebrates
- Frog: Xenopus[36] (X. laevis and X. tropicalis).[37][38] Good embryo supply. Especially suitable for microsurgery.
- Zebrafish: Danio rerio.[39] Good embryo supply. Well developed genetics.
- Chicken: Gallus gallus.[40] Early stages similar to mammal, but microsurgery easier. Low cost.
- Mouse: Mus musculus.[41] A mammal[36] with well developed genetics.
Invertebrates
Unicellular
- Algae: Chlamydomonas[36]
- Yeast: Saccharomyces[36]
Others
Also popular for some purposes have been sea urchins[44][36] and ascidians.[45] For studies of regeneration urodele amphibians such as the axolotl Ambystoma mexicanum are used,[46] and also planarian worms such as Schmidtea mediterranea.[7] Organoids have also been demonstrated as an efficient model for development.[47] Plant development has focused on the thale cress Arabidopsis thaliana as a model organism.[48]...
See also
- Blastocyst
- Body plan
- Cell signaling
- Cell signaling networks
- Embryology
- Enhancer
- Fish development
- Gene regulatory network
- Ontogeny
- Plant evolutionary developmental biology
- Promoter (biology)
- Signal transduction
- Teratology
References
- Li B, Carey M, Workman JL (February 2007). "The role of chromatin during transcription". Cell. 128 (4): 707–19. doi:10.1016/j.cell.2007.01.015. PMID 17320508.
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, et al. (March 2007). "Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome". Nature Genetics. 39 (3): 311–8. doi:10.1038/ng1966. PMID 17277777. S2CID 1595885.
- Meinhardt H, Gierer A (2000). "Pattern formation by local self-activation and lateral inhibition" (PDF). BioEssays. 22 (8): 753–760. CiteSeerX 10.1.1.477.439. doi:10.1002/1521-1878(200008)22:8<753::aid-bies9>3.0.co;2-z. PMID 10918306. Archived (PDF) from the original on 2017-10-27.
- Sprinzak D, Lakhanpal A, Lebon L, Santat LA, Fontes ME, Anderson GA, et al. (May 2010). "Cis-interactions between Notch and Delta generate mutually exclusive signalling states". Nature. 465 (7294): 86–90. Bibcode:2010Natur.465...86S. doi:10.1038/nature08959. PMC 2886601. PMID 20418862.
- Carlson BM (2007). Principles of Regenerative Biology. Burlington MA: Academic Press.
- Bosch TC (March 2007). "Why polyps regenerate and we don't: towards a cellular and molecular framework for Hydra regeneration". Developmental Biology. 303 (2): 421–33. doi:10.1016/j.ydbio.2006.12.012. PMID 17234176.
- Reddien PW, Sánchez Alvarado A (2004). "Fundamentals of planarian regeneration". Annual Review of Cell and Developmental Biology. 20: 725–57. doi:10.1146/annurev.cellbio.20.010403.095114. PMID 15473858. S2CID 1320382.
- Wagner DE, Wang IE, Reddien PW (May 2011). "Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration". Science. 332 (6031): 811–6. Bibcode:2011Sci...332..811W. doi:10.1126/science.1203983. PMC 3338249. PMID 21566185.
- Nakamura T, Mito T, Bando T, Ohuchi H, Noji S (January 2008). "Dissecting insect leg regeneration through RNA interference". Cellular and Molecular Life Sciences. 65 (1): 64–72. doi:10.1007/s00018-007-7432-0. PMID 18030418.
- Simon A, Tanaka EM (2013). "Limb regeneration". Wiley Interdisciplinary Reviews. Developmental Biology. 2 (2): 291–300. doi:10.1002/wdev.73. PMID 24009038. S2CID 13158705.
- Slack JM (2013). "Chapter 20". Essential Developmental Biology. Oxford: Wiley-Blackwell.
- Jungnickel MK, Sutton KA, Florman HM (August 2003). "In the beginning: lessons from fertilization in mice and worms". Cell. 114 (4): 401–4. doi:10.1016/s0092-8674(03)00648-2. PMID 12941269.
- Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, Surani MA (January 2013). "Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine". Science. 339 (6118): 448–52. Bibcode:2013Sci...339..448H. doi:10.1126/science.1229277. PMC 3847602. PMID 23223451.
- Hajkova P, Jeffries SJ, Lee C, Miller N, Jackson SP, Surani MA (July 2010). "Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway". Science. 329 (5987): 78–82. Bibcode:2010Sci...329...78H. doi:10.1126/science.1187945. PMC 3863715. PMID 20595612.
- Steven DH, ed. (1975). Comparative Placentation. London: Academic Press.
- Kimelman D, Martin BL (2012). "Anterior-posterior patterning in early development: three strategies". Wiley Interdisciplinary Reviews. Developmental Biology. 1 (2): 253–66. doi:10.1002/wdev.25. PMC 5560123. PMID 23801439.
- Slack JM (1987). "Morphogenetic gradients - past and present". Trends in Biochemical Sciences. 12: 200–204. doi:10.1016/0968-0004(87)90094-6.
- Rogers KW, Schier AF (2011). "Morphogen gradients: from generation to interpretation". Annual Review of Cell and Developmental Biology. 27: 377–407. doi:10.1146/annurev-cellbio-092910-154148. PMID 21801015. S2CID 21477124.
- Dahmann C, Oates AC, Brand M (January 2011). "Boundary formation and maintenance in tissue development". Nature Reviews. Genetics. 12 (1): 43–55. doi:10.1038/nrg2902. PMID 21164524. S2CID 1805261.
- Hardin J, Walston T (August 2004). "Models of morphogenesis: the mechanisms and mechanics of cell rearrangement". Current Opinion in Genetics & Development. 14 (4): 399–406. doi:10.1016/j.gde.2004.06.008. PMID 15261656.
- Hammerschmidt M, Wedlich D (November 2008). "Regulated adhesion as a driving force of gastrulation movements". Development. 135 (22): 3625–41. doi:10.1242/dev.015701. PMID 18952908.
- O'Farrell PH (2003). "How metazoans reach their full size: the natural history of bigness.". In Hall MN, Raff M, Thomas G (eds.). Cell Growth: Control of Cell Size. Cold Spring Harbor Laboratory Press. pp. 1–21.
- Moss EG, Romer-Seibert J (2014). "Cell-intrinsic timing in animal development". Wiley Interdisciplinary Reviews. Developmental Biology. 3 (5): 365–77. doi:10.1002/wdev.145. PMID 25124757. S2CID 29029979.
- Tata JR (1996). "Amphibian metamorphosis: an exquisite model for hormonal regulation of postembryonic development in vertebrates". Development, Growth and Differentiation. 38 (3): 223–231. doi:10.1046/j.1440-169x.1996.t01-2-00001.x. S2CID 84081060.
- Brown DD, Cai L (June 2007). "Amphibian metamorphosis". Developmental Biology. 306 (1): 20–33. doi:10.1016/j.ydbio.2007.03.021. PMC 1945045. PMID 17449026.
- Cohen SM (1993). "Imaginal Disc Development.". In Bate M, Martinez-Arias M (eds.). The Development of Drosophila melanogaster. Cold Spring Harbor Press.
- Maves L, Schubiger G (October 2003). "Transdetermination in Drosophila imaginal discs: a model for understanding pluripotency and selector gene maintenance". Current Opinion in Genetics & Development. 13 (5): 472–9. doi:10.1016/j.gde.2003.08.006. PMID 14550411.
- Bäurle I, Laux T (October 2003). "Apical meristems: the plant's fountain of youth". Review. BioEssays. 25 (10): 961–70. doi:10.1002/bies.10341. PMID 14505363.
- Leopold AC (1964). Plant Growth and Development. New York: McGraw-Hill. p. 183.
- Brand U, Hobe M, Simon R (February 2001). "Functional domains in plant shoot meristems". Review. BioEssays. 23 (2): 134–41. doi:10.1002/1521-1878(200102)23:2<134::AID-BIES1020>3.0.CO;2-3. PMID 11169586. S2CID 5833219.
- Barlow P (May 2005). "Patterned cell determination in a plant tissue: the secondary phloem of trees". BioEssays. 27 (5): 533–41. doi:10.1002/bies.20214. PMID 15832381.
- Pacifici E, Di Mambro R, Dello Ioio R, Costantino P, Sabatini S (August 2018). "Arabidopsis root". The EMBO Journal. 37 (16). doi:10.15252/embj.201899134. PMC 6092616. PMID 30012836.
- Ross SD, Pharis RP, Binder WD (1983). "Growth regulators and conifers: their physiology and potential uses in forestry.". In Nickell LG (ed.). Plant growth regulating chemicals. Vol. 2. Boca Raton, FL: CRC Press. pp. 35–78.
- Jin J, He K, Tang X, Li Z, Lv L, Zhao Y, et al. (July 2015). "An Arabidopsis Transcriptional Regulatory Map Reveals Distinct Functional and Evolutionary Features of Novel Transcription Factors". Molecular Biology and Evolution. 32 (7): 1767–73. doi:10.1093/molbev/msv058. PMC 4476157. PMID 25750178. Archived from the original on 2016-06-02.
- Pires, Nuno D.; Dolan, Liam (2012-02-19). "Morphological evolution in land plants: new designs with old genes". Philosophical Transactions of the Royal Society B: Biological Sciences. 367 (1588): 508–518. doi:10.1098/rstb.2011.0252. ISSN 0962-8436. PMC 3248709. PMID 22232763.
- Friedman, William E. (1999). "Expression of the cell cycle in sperm of Arabidopsis: implications for understanding patterns of gametogenesis and fertilization in plants and other eukaryotes". Development. The Company of Biologists. 126 (5): 1065–75. doi:10.1242/dev.126.5.1065. ISSN 0950-1991. PMID 9927606. S2CID 13397345.
- Nieuwkoop PD, Faber J (1967). Normal table of Xenopus laevis (Daudin). North-Holland, Amsterdam.
- Harland RM, Grainger RM (December 2011). "Xenopus research: metamorphosed by genetics and genomics". Trends in Genetics. 27 (12): 507–15. doi:10.1016/j.tig.2011.08.003. PMC 3601910. PMID 21963197.
- Lawson ND, Wolfe SA (July 2011). "Forward and reverse genetic approaches for the analysis of vertebrate development in the zebrafish". Developmental Cell. 21 (1): 48–64. doi:10.1016/j.devcel.2011.06.007. PMID 21763608.
- Rashidi H, Sottile V (April 2009). "The chick embryo: hatching a model for contemporary biomedical research". BioEssays. 31 (4): 459–65. doi:10.1002/bies.200800168. PMID 19274658. S2CID 5489431.
- Behringer R, Gertsenstein M, Vintersten K, Nagy M (2014). Manipulating the Mouse Embryo. A Laboratory Manual (Fourth ed.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- St Johnston D (March 2002). "The art and design of genetic screens: Drosophila melanogaster". Nature Reviews. Genetics. 3 (3): 176–88. doi:10.1038/nrg751. PMID 11972155. S2CID 195368351.
- Riddle DL, Blumenthal T, Meyer BJ, Priess JR (1997). C.elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Ettensohn CA, Sweet HC (2000). "Patterning the early sea urchin embryo". Current Topics in Developmental Biology Volume 50. Curr. Top. Dev. Biol. Current Topics in Developmental Biology. Vol. 50. Academic Press. pp. 1–44. doi:10.1016/S0070-2153(00)50002-7. ISBN 9780121531508. PMID 10948448.
- Lemaire P (June 2011). "Evolutionary crossroads in developmental biology: the tunicates". Development. 138 (11): 2143–52. doi:10.1242/dev.048975. PMID 21558365.
- Nacu E, Tanaka EM (2011). "Limb regeneration: a new development?". Annual Review of Cell and Developmental Biology. 27: 409–40. doi:10.1146/annurev-cellbio-092910-154115. PMID 21801016.
- Ader M, Tanaka EM (December 2014). "Modeling human development in 3D culture". Current Opinion in Cell Biology. 31: 23–8. doi:10.1016/j.ceb.2014.06.013. PMID 25033469.
- Weigel D, Glazebrook J (2002). Arabidopsis. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Further reading
- Gilbert SF (2013). Developmental Biology. Sunderland, Mass.: Sinauer Associates Inc.
- Slack JM (2013). Essential Developmental Biology. Oxford: Wiley-Blackwell.
- Wolpert L, Tickle C (2011). Principles of Development. Oxford and New York: Oxford University Press.