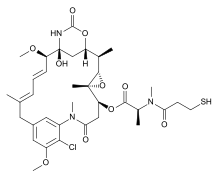
Mertansine
Mertansine, also called DM1 (and in some of its forms emtansine), is a thiol-containing maytansinoid that for therapeutic purposes is attached to a monoclonal antibody through reaction of the thiol group with a linker structure to create an antibody-drug conjugate (ADC).
 | |
| Names | |
|---|---|
| Other names
Maytansinoid DM1 N2'-deacetyl-N2'-(3-mercapto-1-oxopropyl)-maytansine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.168.831 |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C35H48ClN3O10S |
| Molar mass | 738.29 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
ADCs with this design include trastuzumab emtansine, lorvotuzumab mertansine, and cantuzumab mertansine. Some are still experimental; others are in regular clinical use.
Mechanism of action
Mertansine is a tubulin inhibitor, meaning that it inhibits the assembly of microtubules by binding to tubulin (at the rhizoxin binding site).[1]
The monoclonal antibody binds specifically to a structure (usually a protein) occurring in a tumour, thus directing mertansine into this tumour. This concept is called targeted therapy.
Uses and chemistry
The following (experimental) drugs are antibody-drug conjugates (ADC) combining monoclonal antibodies with mertansine as the cytotoxic component. Mertansine is linked via 4-mercaptovaleric acid.[2]
ADCs include:
- Bivatuzumab mertansine
- Cantuzumab mertansine[3]
- Lorvotuzumab mertansine (IMGN901) for CD56 positive cancers, for example multiple myeloma[4]
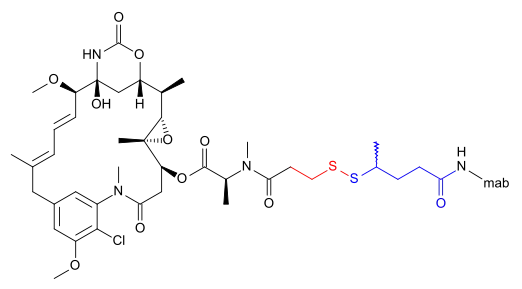
Emtansine
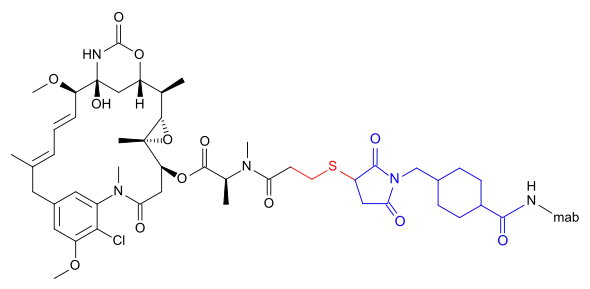
DM1 can also be linked via a more complicated structure – 4-(3-mercapto-2,5-dioxo-1-pyrrolidinylmethyl)-cylohexanecarboxylic acid or SMCC –, in which case the International Nonproprietary Name of the conjugate formed contains the word emtansine. The abbreviation comes from the chemical designation "succinimidyl-trans-4-(maleimidylmethyl) cyclohexane-1-carboxylate" which is used in the primary literature[5] as well as by the World Health Organization (WHO)[6] despite the fact that the linker contains only one imide group according to the WHO.[2]
DM1 and its attachment via these linkers result from ImmunoGen Inc research.
An example is:
- Trastuzumab emtansine (T-DM1), an anti-HER2/neu antibody-drug conjugate[7][8]
References
- National Cancer Institute: Definition of Maytansine
- "International Nonproprietary Names (INN) for pharmaceutical substances: Names for radicals, groups & others" (PDF). WHO. 2012: 66f.
{{cite journal}}: Cite journal requires|journal=(help) - "International Nonproprietary Names for Pharmaceutical Substances (INN): List 89" (PDF). WHO. 2003: 188.
{{cite journal}}: Cite journal requires|journal=(help) - "ImmunoGen reports encouraging clinical data of IMGN901". The Medical News. 6 December 2009.
- Girish, Sandhya; Gupta, Manish; Wang, Bei; Lu, Dan; Krop, Ian E.; Vogel, Charles L.; Burris Iii, Howard A.; Lorusso, Patricia M.; Yi, Joo-Hee; Saad, Ola; Tong, Barbara; Chu, Yu-Waye; Holden, Scott; Joshi, Amita (May 2012). "Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody–drug conjugate in development for the treatment of HER2-positive cancer". Cancer Chemother Pharmacol. 69 (5): 1229–1240. doi:10.1007/s00280-011-1817-3. PMC 3337408. PMID 22271209.
- "International Nonproprietary Names for Pharmaceutical Substances (INN): List 103" (PDF). WHO. 2010: 172.
{{cite journal}}: Cite journal requires|journal=(help) - National Cancer Institute: trastuzumab-MCC-DM1 antibody-drug conjugate
- ImmunoGen: Trastuzumab-DM1 Archived 2010-10-20 at the Wayback Machine