Galactose-1-phosphate uridylyltransferase
Galactose-1-phosphate uridylyltransferase (or GALT, G1PUT) is an enzyme (EC 2.7.7.12) responsible for converting ingested galactose to glucose.[5]
| GALT | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | GALT, entrez:2592, galactose-1-phosphate uridylyltransferase | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 606999 MGI: 95638 HomoloGene: 126 GeneCards: GALT | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Galactose-1-phosphate uridyl transferase, N-terminal domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | GalP_UDP_transf | ||||||||
| Pfam | PF01087 | ||||||||
| Pfam clan | CL0265 | ||||||||
| PROSITE | PDOC00108 | ||||||||
| SCOP2 | 1hxp / SCOPe / SUPFAM | ||||||||
| |||||||||
| Galactose-1-phosphate uridyl transferase, C-terminal domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 structure of nucleotidyltransferase complexed with udp-galactose | |||||||||
| Identifiers | |||||||||
| Symbol | GalP_UDP_tr_C | ||||||||
| Pfam | PF02744 | ||||||||
| Pfam clan | CL0265 | ||||||||
| InterPro | IPR005850 | ||||||||
| PROSITE | PDOC00108 | ||||||||
| SCOP2 | 1hxp / SCOPe / SUPFAM | ||||||||
| |||||||||
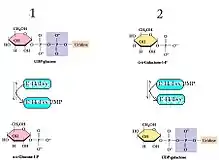
Galactose-1-phosphate uridylyltransferase (GALT) catalyzes the second step of the Leloir pathway of galactose metabolism, namely:
- UDP-glucose + galactose 1-phosphate glucose 1-phosphate + UDP-galactose
The expression of GALT is controlled by the actions of the FOXO3 gene. The absence of this enzyme results in classic galactosemia in humans and can be fatal in the newborn period if lactose is not removed from the diet. The pathophysiology of galactosemia has not been clearly defined.[5]
 galactose
galactose
Mechanism
GALT catalyzes the second reaction of the Leloir pathway of galactose metabolism through ping pong bi-bi kinetics with a double displacement mechanism.[6] This means that the net reaction consists of two reactants and two products (see the reaction above) and it proceeds by the following mechanism: the enzyme reacts with one substrate to generate one product and a modified enzyme, which goes on to react with the second substrate to make the second product while regenerating the original enzyme.[7] In the case of GALT, the His166 residue acts as a potent nucleophile to facilitate transfer of a nucleotide between UDP-hexoses and hexose-1-phosphates.[8]
- UDP-glucose + E-His ⇌ Glucose-1-phosphate + E-His-UMP
- Galactose-1-phosphate + E-His-UMP ⇌ UDP-galactose + E-His[8]
Structural studies
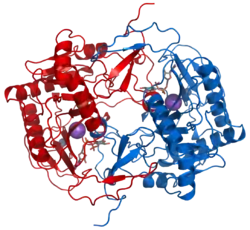
The three-dimensional structure at 180 pm resolution (x-ray crystallography) of GALT was determined by Wedekind, Frey, and Rayment, and their structural analysis found key amino acids essential for GALT function.[8] Among these are Leu4, Phe75, Asn77, Asp78, Phe79, and Val108, which are consistent with residues that have been implicated both in point mutation experiments as well as in clinical screening that play a role in human galactosemia.[8][10]
Clinical significance
Deficiency of GALT causes classic galactosemia. Galactosemia is an autosomal recessive inherited disorder detectable in newborns and childhood.[11] It occurs at approximately 1 in every 40,000-60,000 live-born infants. Classical galactosemia (G/G) is caused by a deficiency in GALT activity, whereas the more common clinical manifestations, Duarte (D/D) and the Duarte/Classical variant (D/G) are caused by the attenuation of GALT activity.[12] Symptoms include ovarian failure, developmental coordination disorder (difficulty speaking correctly and consistently),[13] and neurologic deficits.[12] A single mutation in any of several base pairs can lead to deficiency in GALT activity.[14] For example, a single mutation from A to G in exon 6 of the GALT gene changes Glu188 to an arginine and a mutation from A to G in exon 10 converts Asn314 to an aspartic acid.[12] These two mutations also add new restriction enzyme cut sites, which enable detection by and large-scale population screening with PCR (polymerase chain reaction).[12] Screening has mostly eliminated neonatal death by G/G galactosemia, but the disease, due to GALT’s role in the biochemical metabolism of ingested galactose (which is toxic when accumulated) to the energetically useful glucose, can certainly be fatal.[11][15] However, those afflicted with galactosemia can live relatively normal lives by avoiding milk products and anything else containing galactose (because it cannot be metabolized), but there is still the potential for problems in neurological development or other complications, even in those who avoid galactose.[16]
Disease database
References
- GRCh38: Ensembl release 89: ENSG00000213930 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000036073 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: GALT galactose-1-phosphate uridylyltransferase".
- Wong LJ, Frey PA (September 1974). "Galactose-1-phosphate uridylyltransferase: rate studies confirming a uridylyl-enzyme intermediate on the catalytic pathway". Biochemistry. 13 (19): 3889–94. doi:10.1021/bi00716a011. PMID 4606575.
- "Double displacement mechanism - Definition". Archived from the original on 2016-03-03. Retrieved 2010-05-19.
- Wedekind JE, Frey PA, Rayment I (September 1995). "Three-dimensional structure of galactose-1-phosphate uridylyltransferase from Escherichia coli at 1.8 A resolution". Biochemistry. 34 (35): 11049–61. doi:10.1021/bi00035a010. PMID 7669762.
- "Untitled Document". Archived from the original on 2008-12-04. Retrieved 2010-05-19.
- Seyrantepe V, Ozguc M, Coskun T, Ozalp I, Reichardt JK (1999). "Identification of mutations in the galactose-1-phosphate uridyltransferase (GALT) gene in 16 Turkish patients with galactosemia, including a novel mutation of F294Y. Mutation in brief no. 235. Online". Human Mutation. 13 (4): 339. doi:10.1002/(SICI)1098-1004(1999)13:4<339::AID-HUMU18>3.0.CO;2-S. PMID 10220154.
- Fridovich-Keil JL (December 2006). "Galactosemia: the good, the bad, and the unknown". Journal of Cellular Physiology. 209 (3): 701–5. doi:10.1002/jcp.20820. PMID 17001680. S2CID 32233614.
- Elsas LJ, Langley S, Paulk EM, Hjelm LN, Dembure PP (1995). "A molecular approach to galactosemia". European Journal of Pediatrics. 154 (7 Suppl 2): S21-7. doi:10.1007/BF02143798. PMID 7671959. S2CID 11937698.
- "Apraxia of Speech". Archived from the original on 2006-02-28. Retrieved 2010-05-19.
- Dobrowolski SF, Banas RA, Suzow JG, Berkley M, Naylor EW (February 2003). "Analysis of common mutations in the galactose-1-phosphate uridyl transferase gene: new assays to increase the sensitivity and specificity of newborn screening for galactosemia". The Journal of Molecular Diagnostics. 5 (1): 42–7. doi:10.1016/S1525-1578(10)60450-3. PMC 1907369. PMID 12552079.
- Lai K, Elsas LJ, Wierenga KJ (November 2009). "Galactose toxicity in animals". IUBMB Life. 61 (11): 1063–74. doi:10.1002/iub.262. PMC 2788023. PMID 19859980.
- "Galactosemia - Treatment". Archived from the original on 2002-08-28.
Further reading
- Reichardt JK (1993). "Genetic basis of galactosemia". Human Mutation. 1 (3): 190–6. doi:10.1002/humu.1380010303. PMID 1301925. S2CID 504197.
- Tyfield L, Reichardt J, Fridovich-Keil J, Croke DT, Elsas LJ, Strobl W, Kozak L, Coskun T, Novelli G, Okano Y, Zekanowski C, Shin Y, Boleda MD (1999). "Classical galactosemia and mutations at the galactose-1-phosphate uridyl transferase (GALT) gene". Human Mutation. 13 (6): 417–30. doi:10.1002/(SICI)1098-1004(1999)13:6<417::AID-HUMU1>3.0.CO;2-0. PMID 10408771. S2CID 34860932.
- Reichardt JK, Belmont JW, Levy HL, Woo SL (March 1992). "Characterization of two missense mutations in human galactose-1-phosphate uridyltransferase: different molecular mechanisms for galactosemia". Genomics. 12 (3): 596–600. doi:10.1016/0888-7543(92)90453-Y. PMID 1373122.
- Leslie ND, Immerman EB, Flach JE, Florez M, Fridovich-Keil JL, Elsas LJ (October 1992). "The human galactose-1-phosphate uridyltransferase gene". Genomics. 14 (2): 474–80. doi:10.1016/S0888-7543(05)80244-7. PMID 1427861.
- Reichardt JK, Levy HL, Woo SL (June 1992). "Molecular characterization of two galactosemia mutations and one polymorphism: implications for structure-function analysis of human galactose-1-phosphate uridyltransferase". Biochemistry. 31 (24): 5430–3. doi:10.1021/bi00139a002. PMID 1610789.
- Reichardt JK, Packman S, Woo SL (October 1991). "Molecular characterization of two galactosemia mutations: correlation of mutations with highly conserved domains in galactose-1-phosphate uridyl transferase". American Journal of Human Genetics. 49 (4): 860–7. PMC 1683190. PMID 1897530.
- Reichardt JK, Woo SL (April 1991). "Molecular basis of galactosemia: mutations and polymorphisms in the gene encoding human galactose-1-phosphate uridylyltransferase". Proceedings of the National Academy of Sciences of the United States of America. 88 (7): 2633–7. Bibcode:1991PNAS...88.2633R. doi:10.1073/pnas.88.7.2633. PMC 51292. PMID 2011574.
- Flach JE, Reichardt JK, Elsas LJ (August 1990). "Sequence of a cDNA encoding human galactose-1-phosphate uridyl transferase". Molecular Biology & Medicine. 7 (4): 365–9. PMID 2233247.
- Reichardt JK, Berg P (April 1988). "Cloning and characterization of a cDNA encoding human galactose-1-phosphate uridyl transferase". Molecular Biology & Medicine. 5 (2): 107–22. PMID 2840550.
- Bergren WG, Donnell GN (July 1973). "A new variant of galactose-1-phosphate uridyltransferase in man: the Los Angeles variant". Annals of Human Genetics. 37 (1): 1–8. doi:10.1111/j.1469-1809.1973.tb01808.x. PMID 4759900. S2CID 22699183.
- Shih LY, Suslak L, Rosin I, Searle BM, Desposito F (November 1984). "Gene dosage studies supporting localization of the structural gene for galactose-1-phosphate uridyl transferase (GALT) to band p13 of chromosome 9". American Journal of Medical Genetics. 19 (3): 539–43. doi:10.1002/ajmg.1320190316. PMID 6095663.
- Ashino J, Okano Y, Suyama I, Yamazaki T, Yoshino M, Furuyama J, Lin HC, Reichardt JK, Isshiki G (1995). "Molecular characterization of galactosemia (type 1) mutations in Japanese". Human Mutation. 6 (1): 36–43. doi:10.1002/humu.1380060108. PMID 7550229. S2CID 23500152.
- Elsas LJ, Langley S, Paulk EM, Hjelm LN, Dembure PP (1995). "A molecular approach to galactosemia". European Journal of Pediatrics. 154 (7 Suppl 2): S21-7. doi:10.1007/BF02143798. PMID 7671959. S2CID 11937698.
- Elsas LJ, Langley S, Steele E, Evinger J, Fridovich-Keil JL, Brown A, Singh R, Fernhoff P, Hjelm LN, Dembure PP (March 1995). "Galactosemia: a strategy to identify new biochemical phenotypes and molecular genotypes". American Journal of Human Genetics. 56 (3): 630–9. PMC 1801164. PMID 7887416.
- Fridovich-Keil JL, Langley SD, Mazur LA, Lennon JC, Dembure PP, Elsas JL (March 1995). "Identification and functional analysis of three distinct mutations in the human galactose-1-phosphate uridyltransferase gene associated with galactosemia in a single family". American Journal of Human Genetics. 56 (3): 640–6. PMC 1801186. PMID 7887417.
- Davit-Spraul A, Pourci ML, Ng KH, Soni T, Lemonnier A (November 1994). "Regulatory effects of galactose on galactose-1-phosphate uridyltransferase activity on human hepatoblastoma HepG2 cells". FEBS Letters. 354 (2): 232–6. doi:10.1016/0014-5793(94)01133-8. PMID 7957929. S2CID 45242645.
- Lin HC, Kirby LT, Ng WG, Reichardt JK (February 1994). "On the molecular nature of the Duarte variant of galactose-1-phosphate uridyl transferase (GALT)". Human Genetics. 93 (2): 167–9. doi:10.1007/BF00210604. PMID 8112740. S2CID 42558872.
- Elsas LJ, Dembure PP, Langley S, Paulk EM, Hjelm LN, Fridovich-Keil J (June 1994). "A common mutation associated with the Duarte galactosemia allele". American Journal of Human Genetics. 54 (6): 1030–6. PMC 1918187. PMID 8198125.
- Reichardt JK, Novelli G, Dallapiccola B (March 1993). "Molecular characterization of the H319Q galactosemia mutation". Human Molecular Genetics. 2 (3): 325–6. doi:10.1093/hmg/2.3.325. PMID 8499924.
External links
- Galactose-1-P-Uridyltransferase at the US National Library of Medicine Medical Subject Headings (MeSH)
- GeneReviews/NIH/NCBI/UW entry on Galactosemia
- Galactosemia (GALT) Mutation Database
- GALT Protein Database
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Galactose-1-phosphate uridylyltransferase