HCN channel
Hyperpolarization-activated cyclic nucleotide–gated (HCN) channels are integral membrane proteins that serve as nonselective voltage-gated cation channels in the plasma membranes of heart and brain cells.[1] HCN channels are sometimes referred to as pacemaker channels because they help to generate rhythmic activity within groups of heart and brain cells. HCN channels are activated by membrane hyperpolarization, are permeable to Na + and K +, and are constitutively open at voltages near the resting membrane potential.[2] HCN channels are encoded by four genes (HCN1, 2, 3, 4) and are widely expressed throughout the heart and the central nervous system.[3][4]
The current through HCN channels, designated If or Ih, plays a key role in the control of cardiac and neuronal rhythmicity and is called the pacemaker current or "funny" current. Expression of single isoforms in heterologous systems such as human embryonic kidney (HEK) cells, Chinese hamster ovary (CHO) cells and Xenopus oocytes yield homotetrameric channels able to generate ion currents with properties similar to those of the native If/Ih current, but with quantitative differences in the voltage-dependence, activation/deactivation kinetics and sensitivity to the nucleotide cyclic AMP (cAMP): HCN1 channels have a more positive threshold for activation, faster activation kinetics, and a lower sensitivity to cAMP, while HCN4 channels are slowly gating and strongly sensitive to cAMP. HCN2 and HCN3 have intermediate properties.[5][6][7]
Structure
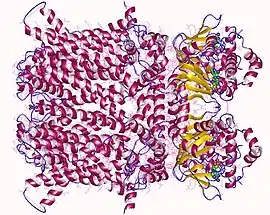
Hyperpolarization-activated and cyclic nucleotide–gated (HCN) channels belong to the superfamily of voltage-gated K+ (Kv) and cyclic nucleotide–gated (CNG) channels. HCN channels are thought to consist of four either identical or non-identical subunits that are integrally embedded in the cell membrane to create an ion-conducting pore.[8] Each subunit comprises six membrane-spanning (S1–6) domains which include a putative voltage sensor (S4) and a pore region between S5 and S6 carrying the GYG triplet signature of K+-permeable channels, and a cyclic nucleotide-binding domain (CNBD) in the C-terminus. HCN isoforms are highly conserved in their core transmembrane regions and cyclic nucleotide binding domain (80–90% identical), but diverge in their amino- and carboxy-terminal cytoplasmic regions.[6]
HCN channels are regulated by both intracellular and extracellular molecules, but most importantly, by cyclic nucleotides (cAMP, cGMP, cCMP).[9][10][11] Binding of cyclic nucleotides lowers the threshold potential of HCN channels, thus activating them. cAMP is a primary agonist of HCN2 while cGMP and cCMP may also bind to it. All three, however, are potent agonists.[12]
Cardiac function
HCN4 is the main isoform expressed in the sinoatrial node, but low levels of HCN1 and HCN2 have also been reported. The current through HCN channels, called the pacemaker current (If), plays a key role in the generation and modulation of cardiac rhythmicity,[13] as they are responsible for the spontaneous depolarization in pacemaker action potentials in the heart. HCN4 isoforms are regulated by cCMP and cAMP and these molecules are agonists at If.[14][15]
Function in the nervous system
All four HCN subunits are expressed in the brain.[4] In addition to their proposed roles in pacemaking rhythmic or oscillatory activity, HCN channels may control the way that neurons respond to synaptic input. Initial studies suggest roles for HCN channels in sour taste, coordinated motor behavior and aspects of learning and memory. Clinically, there is evidence that HCN channels play roles in epilepsy and neuropathic pain. HCN channels have been shown to be important for activity-dependent mechanisms for olfactory sensory neuron growth.[16]
HCN1 and 2 channels have been found in dorsal root ganglia, basal ganglia, and the dendrites of neurons in the hippocampus. It has been found that human cortical neurons have particularly high amount of HCN1 channel expression in all layers.[17] HCN channel trafficking along dendrites in the hippocampus of rats has shown that HCN channels are quickly shuttled to the surface in response to neural activity.[18] HCN channels have also been observed in the retrotrapezoid nucleus (RTN), a respiratory control center that responds to chemical signals such as CO2. When HCN is inhibited, serotonin fails to stimulate chemoreceptors in the RTN. This illustrates a connection between HCN channels and respiratory regulation.[19] Due to the complex nature of HCN channel regulation, as well as the complex interactions between multiple ion channels, HCN channels are fine-tuned to respond to certain thresholds and agonists. This complexity is believed to affect neural plasticity.[18]
History
HCN channel was first identified in 1976 in the heart by Noma and Irisawa and characterized by Brown, Difrancesco and Weiss [20]
See also
References
- Luthi A, McCormick DA. 1998. Neuron. H-current: properties of a neuronal and network pacemaker. Vol. 21. pp 9-12.
- Benarroch EE. HCN channels: function and clinical implications. Neurology. 2013 Jan 15;80(3):304-10. doi: 10.1212/WNL.0b013e31827dec42. PMID 23319474.
- Kaupp UB, Seifert R. Molecular diversity of pacemaker ion channels (2001) Annu Rev Physiol. 63:235-57. Review.
- Notomi, T; Shigemoto, R (2004). "Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain". J Comp Neurol. 471 (3): 241–276. doi:10.1002/cne.11039. PMID 14991560. S2CID 12236560.
- Wahl-Schott, C; Biel, M (Feb 2009). "HCN channels: structure, cellular regulation and physiological function". Cell Mol Life Sci. 66 (3): 470–94. doi:10.1007/s00018-008-8525-0. PMID 18953682. S2CID 12774911.
- Baruscotti, M.; Bucchi, A.; DiFrancesco, D. (2005). "Physiology and pharmacology of the cardiac pacemaker ("funny") current". Pharmacology & Therapeutics. 107 (1): 59–79. doi:10.1016/j.pharmthera.2005.01.005. PMID 15963351.
- Santoro, B; Tibbs, GR (1999). "The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels". Ann N Y Acad Sci. 868 (1): 741–64. Bibcode:1999NYASA.868..741S. doi:10.1111/j.1749-6632.1999.tb11353.x. PMID 10414361. S2CID 38066720.
- "Swiss-Prot entry". Archived from the original on 2011-07-27. Retrieved 2008-04-15.
- He, Chao; Chen, Fang; Li, Bo; Hu, Zhian (2014). "Neurophysiology of HCN channels: From cellular functions to multiple regulations". Progress in Neurobiology. 112: 1–23. doi:10.1016/j.pneurobio.2013.10.001. PMID 24184323. S2CID 37519503.
- Mishra, Poonam; Narayanan, Rishikesh (2015-01-01). "High-conductance states and A-type K+ channels are potential regulators of the conductance-current balance triggered by HCN channels". Journal of Neurophysiology. 113 (1): 23–43. doi:10.1152/jn.00601.2013. ISSN 0022-3077. PMID 25231614.
- Neymotin, S.A.; McDougal, R.A.; Bulanova, A.S.; Zeki, M.; Lakatos, P.; Terman, D.; Hines, M.L.; Lytton, W.W. (2016). "Calcium regulation of HCN channels supports persistent activity in a multiscale model of neocortex". Neuroscience. 316: 344–366. doi:10.1016/j.neuroscience.2015.12.043. PMC 4724569. PMID 26746357.
- DeBerg, Hannah A.; Brzovic, Peter S.; Flynn, Galen E.; Zagotta, William N.; Stoll, Stefan (2016-01-01). "Structure and Energetics of Allosteric Regulation of HCN2 Ion Channels by Cyclic Nucleotides". Journal of Biological Chemistry. 291 (1): 371–381. doi:10.1074/jbc.m115.696450. ISSN 0021-9258. PMC 4697172. PMID 26559974.
- Larsson, H. P. (2010). "How is the heart rate regulated in the sinoatrial node? Another piece to the puzzle". The Journal of General Physiology. 136 (3): 237–241. doi:10.1085/jgp.201010506. PMC 2931147. PMID 20713549.
- Zong, Xiangang; Krause, Stefanie; Chen, Cheng-Chang; Krüger, Jens; Gruner, Christian; Cao-Ehlker, Xiaochun; Fenske, Stefanie; Wahl-Schott, Christian; Biel, Martin (2012-08-03). "Regulation of Hyperpolarization-activated Cyclic Nucleotide-gated (HCN) Channel Activity by cCMP". Journal of Biological Chemistry. 287 (32): 26506–26512. doi:10.1074/jbc.m112.357129. ISSN 0021-9258. PMC 3410992. PMID 22715094.
- Greene, Derek; Kang, Seungwoo; Kosenko, Anastasia; Hoshi, Naoto (2012-07-06). "Adrenergic Regulation of HCN4 Channel Requires Protein Association with β2-Adrenergic Receptor". Journal of Biological Chemistry. 287 (28): 23690–23697. doi:10.1074/jbc.m112.366955. ISSN 0021-9258. PMC 3390643. PMID 22613709.
- Mobley, AS; Miller, AM; Araneda, RC; Maurer, LR; Müller, F; Greer, CA (8 December 2010). "Hyperpolarization-activated cyclic nucleotide-gated channels in olfactory sensory neurons regulate axon extension and glomerular formation". The Journal of Neuroscience. 30 (49): 16498–508. doi:10.1523/JNEUROSCI.4225-10.2010. PMC 3393111. PMID 21147989.
- Kalmbach, Brian E.; Buchin, Anatoly; Long, Brian; Close, Jennie; Nandi, Anirban; Miller, Jeremy A.; Bakken, Trygve E.; Hodge, Rebecca D.; Chong, Peter (2018-12-05). "h-Channels Contribute to Divergent Intrinsic Membrane Properties of Supragranular Pyramidal Neurons in Human versus Mouse Cerebral Cortex". Neuron. 100 (5): 1194–1208.e5. doi:10.1016/j.neuron.2018.10.012. ISSN 1097-4199. PMC 6447369. PMID 30392798.
- Noam, Yoav; Zha, Qinqin; Phan, Lise; Wu, Rui-Lin; Chetkovich, Dane M.; Wadman, Wytse J.; Baram, Tallie Z. (2010-05-07). "Trafficking and Surface Expression of Hyperpolarization-activated Cyclic Nucleotide-gated Channels in Hippocampal Neurons". Journal of Biological Chemistry. 285 (19): 14724–14736. doi:10.1074/jbc.m109.070391. ISSN 0021-9258. PMC 2863223. PMID 20215108.
- Hawkins, Virginia E.; Hawryluk, Joanna M.; Takakura, Ana C.; Tzingounis, Anastasios V.; Moreira, Thiago S.; Mulkey, Daniel K. (2015-02-15). "HCN channels contribute to serotonergic modulation of ventral surface chemosensitive neurons and respiratory activity". Journal of Neurophysiology. 113 (4): 1195–1205. doi:10.1152/jn.00487.2014. ISSN 0022-3077. PMC 4329434. PMID 25429115.
- Kase, Daisuke; Imoto, Keiji (2012-08-13). "The Role of HCN Channels on Membrane Excitability in the Nervous System". Journal of Signal Transduction. 2012: 619747. doi:10.1155/2012/619747. PMC 3425855. PMID 22934165.
External links
- "Cyclic Nucleotide Regulated Channels". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology.