Hill equation (biochemistry)
In biochemistry and pharmacology, the Hill equation refers to two closely related equations that reflect the binding of ligands to macromolecules, as a function of the ligand concentration. A ligand is "a substance that forms a complex with a biomolecule to serve a biological purpose" (ligand definition), and a macromolecule is a very large molecule, such as a protein, with a complex structure of components (macromolecule definition). Protein-ligand binding typically changes the structure of the target protein, thereby changing its function in a cell.
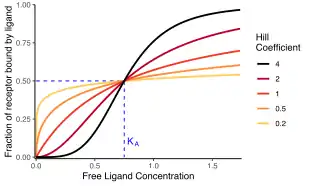
The distinction between the two Hill equations is whether they measure occupancy or response. The Hill–Langmuir equation reflects the occupancy of macromolecules: the fraction that is saturated or bound by the ligand.[1][2][nb 1] This equation is formally equivalent to the Langmuir isotherm.[3] Conversely, the Hill equation proper reflects the cellular or tissue response to the ligand: the physiological output of the system, such as muscle contraction.
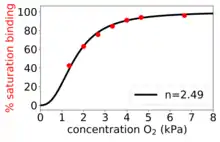
The Hill–Langmuir equation was originally formulated by Archibald Hill in 1910 to describe the sigmoidal O2 binding curve of haemoglobin.[4]
The binding of a ligand to a macromolecule is often enhanced if there are already other ligands present on the same macromolecule (this is known as cooperative binding). The Hill–Langmuir equation is useful for determining the degree of cooperativity of the ligand(s) binding to the enzyme or receptor. The Hill coefficient provides a way to quantify the degree of interaction between ligand binding sites.[5]
The Hill equation (for response) is important in the construction of dose-response curves.
Proportion of ligand-bound receptors
The Hill–Langmuir equation is a special case of a rectangular hyperbola and is commonly expressed in the following ways.[2][7][8]
- ,
where:
- is the fraction of the receptor protein concentration that is bound by the ligand,
- is the total ligand concentration,
- is the apparent dissociation constant derived from the law of mass action,
- is the ligand concentration producing half occupation,
- is the Hill coefficient.
The special case where is a Monod equation.
Constants
In pharmacology, is often written as , where is the ligand, equivalent to L, and is the receptor. can be expressed in terms of the total amount of receptor and ligand-bound receptor concentrations: . is equal to the ratio of the dissociation rate of the ligand-receptor complex to its association rate ().[8] Kd is the equilibrium constant for dissociation. is defined so that , this is also known as the microscopic dissociation constant and is the ligand concentration occupying half of the binding sites. In recent literature, this constant is sometimes referred to as .[8]
Gaddum equation
The Gaddum equation is a further generalisation of the Hill-equation, incorporating the presence of a reversible competitive antagonist.[1] The Gaddum equation is derived similarly to the Hill-equation but with 2 equilibria: both the ligand with the receptor and the antagonist with the receptor. Hence, the Gaddum equation has 2 constants: the equilibrium constants of the ligand and that of the antagonist
Hill plot
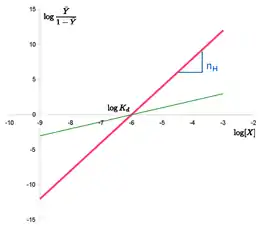
The Hill plot is the rearrangement of the Hill–Langmuir Equation into a straight line.
Taking the reciprocal of both sides of the Hill–Langmuir equation, rearranging, and inverting again yields: . Taking the logarithm of both sides of the equation leads to an alternative formulation of the Hill-Langmuir equation:
- .
This last form of the Hill–Langmuir equation is advantageous because a plot of versus yields a linear plot, which is called a Hill plot.[7][8] Because the slope of a Hill plot is equal to the Hill coefficient for the biochemical interaction, the slope is denoted by . A slope greater than one thus indicates positively cooperative binding between the receptor and the ligand, while a slope less than one indicates negatively cooperative binding.
Transformations of equations into linear forms such as this were very useful before the widespread use of computers, as they allowed researchers to determine parameters by fitting lines to data. However, these transformations affect error propagation, and this may result in undue weight to error in data points near 0 or 1.[nb 2] This impacts the parameters of linear regression lines fitted to the data. Furthermore, the use of computers enables more robust analysis involving nonlinear regression.
Tissue response
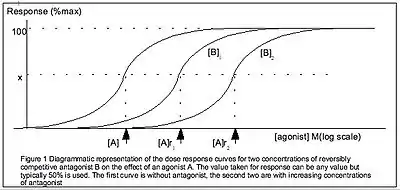
A distinction should be made between quantification of drugs binding to receptors and drugs producing responses. There may not necessarily be a linear relationship between the two values. In contrast to this article's previous definition of the Hill-Langmuir equation, the IUPHAR defines the Hill equation in terms of the tissue response , as[1]
where is the drug concentration and is the drug concentration that produces a 50% maximal response. Dissociation constants (in the previous section) relate to ligand binding, while reflects tissue response.
This form of the equation can reflect tissue/cell/population responses to drugs and can be used to generate dose response curves. The relationship between and EC50 may be quite complex as a biological response will be the sum of myriad factors; a drug will have a different biological effect if more receptors are present, regardless of its affinity.
The Del-Castillo Katz model is used to relate the Hill–Langmuir equation to receptor activation by including a second equilibrium of the ligand-bound receptor to an activated form of the ligand-bound receptor.
Statistical analysis of response as a function of stimulus may be performed by regression methods such as the probit model or logit model, or other methods such as the Spearman–Karber method.[9] Empirical models based on nonlinear regression are usually preferred over the use of some transformation of the data that linearizes the dose-response relationship.[10]
Hill coefficient
The Hill coefficient is a measure of ultrasensitivity (i.e. how steep is the response curve).
The Hill coefficient, or , may describe cooperativity (or possibly other biochemical properties, depending on the context in which the Hill–Langmuir equation is being used). When appropriate, the value of the Hill coefficient describes the cooperativity of ligand binding in the following way:
- . Positively cooperative binding: Once one ligand molecule is bound to the enzyme, its affinity for other ligand molecules increases. For example, the Hill coefficient of oxygen binding to haemoglobin (an example of positive cooperativity) falls within the range of 1.7–3.2.[5]
- . Negatively cooperative binding: Once one ligand molecule is bound to the enzyme, its affinity for other ligand molecules decreases.
- . Noncooperative (completely independent) binding: The affinity of the enzyme for a ligand molecule is not dependent on whether or not other ligand molecules are already bound. When n=1, we obtain a model that can be modeled by Michaelis–Menten kinetics,[11] in which , the Michaelis–Menten constant.
The Hill coefficient can be calculated in terms of potency as:
- .[12]
where and are the input values needed to produce the 10% and 90% of the maximal response, respectively.[13]
Derivation from mass action kinetics
The Hill-Langmuir equation is derived similarly to the Michaelis Menten equation[14][15] but incorporates the Hill coefficient. Consider a protein (), such as haemoglobin or a protein receptor, with binding sites for ligands (). The binding of the ligands to the protein can be represented by the chemical equilibrium expression:
where (forward rate, or the rate of association of the protein-ligand complex) and (reverse rate, or the complex's rate of dissociation) are the reaction rate constants for the association of the ligands to the protein and their dissociation from the protein, respectively.[8] From the law of mass action, which in turn may be derived from the principles of collision theory, the apparent dissociation constant , an equilibrium constant, is given by:
At the same time, , the ratio of the concentration of occupied receptor to total receptor concentration, is given by:
By using the expression obtained earlier for the dissociation constant, we can replace with to yield a simplified expression for :
which is a common formulation of the Hill equation.[7][16][8]
Assuming that the protein receptor was initially completely free (unbound) at a concentration , then at any time, and . Consequently, the Hill–Langmuir Equation is also commonly written as an expression for the concentration of bound protein:
All of these formulations assume that the protein has sites to which ligands can bind. In practice, however, the Hill Coefficient rarely provides an accurate approximation of the number of ligand binding sites on a protein.[5][7] Consequently, it has been observed that the Hill coefficient should instead be interpreted as an "interaction coefficient" describing the cooperativity among ligand binding sites.[5]
Applications
The Hill and Hill–Langmuir equations are used extensively in pharmacology to quantify the functional parameters of a drug and are also used in other areas of biochemistry.
The Hill equation can be used to describe dose-response relationships, for example ion channel open-probability (P-open) vs. ligand concentration.[17]
Regulation of gene transcription
The Hill–Langmuir equation can be applied in modelling the rate at which a gene product is produced when its parent gene is being regulated by transcription factors (e.g., activators and/or repressors).[11] Doing so is appropriate when a gene is regulated by multiple binding sites for transcription factors, in which case the transcription factors may bind the DNA in a cooperative fashion.[18]
If the production of protein from gene X is up-regulated (activated) by a transcription factor Y, then the rate of production of protein X can be modeled as a differential equation in terms of the concentration of activated Y protein:
- ,
where k is the maximal transcription rate of gene X.
Likewise, if the production of protein from gene Y is down-regulated (repressed) by a transcription factor Z, then the rate of production of protein Y can be modeled as a differential equation in terms of the concentration of activated Z protein:
- ,
where k is the maximal transcription rate of gene Y.
Limitations
Because of its assumption that ligand molecules bind to a receptor simultaneously, the Hill–Langmuir equation has been criticized as a physically unrealistic model.[5] Moreover, the Hill coefficient should not be considered a reliable approximation of the number of cooperative ligand binding sites on a receptor[5][19] except when the binding of the first and subsequent ligands results in extreme positive cooperativity.[5]
Unlike more complex models, the relatively simple Hill–Langmuir equation provides little insight into underlying physiological mechanisms of protein-ligand interactions. This simplicity, however, is what makes the Hill–Langmuir equation a useful empirical model, since its use requires little a priori knowledge about the properties of either the protein or ligand being studied.[2] Nevertheless, other, more complex models of cooperative binding have been proposed.[7] For more information and examples of such models, see Cooperative binding.
Global sensitivity measure such as Hill coefficient do not characterise the local behaviours of the s-shaped curves. Instead, these features are well captured by the response coefficient measure.[20]
There is a link between Hill Coefficient and Response coefficient, as follows. Altszyler et al. (2017) have shown that these ultrasensitivity measures can be linked.[12]
See also
- Binding coefficient
- Bjerrum plot
- Cooperative binding
- Gompertz curve
- Langmuir adsorption model
- Logistic function
- Michaelis–Menten kinetics
- Monod equation
Notes
- For clarity, this article will use the International Union of Basic and Clinical Pharmacology convention of distinguishing between the Hill-Langmuir equation (for receptor saturation) and Hill equation (for tissue response)
- See Propagation of uncertainty. The function propagates errors in as . Hence errors in values of near or are given far more weight than those for
References
- Neubig, Richard R. (2003). "International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on Terms and Symbols in Quantitative Pharmacology" (PDF). Pharmacological Reviews. 55 (4): 597–606. doi:10.1124/pr.55.4.4. PMID 14657418. S2CID 1729572.
- Gesztelyi, Rudolf; Zsuga, Judit; Kemeny-Beke, Adam; Varga, Balazs; Juhasz, Bela; Tosaki, Arpad (31 March 2012). "The Hill equation and the origin of quantitative pharmacology". Archive for History of Exact Sciences. 66 (4): 427–438. doi:10.1007/s00407-012-0098-5. ISSN 0003-9519. S2CID 122929930.
- Langmuir, Irving (1918). "The adsorption of gases on plane surfaces of glass, mica and platinum". Journal of the American Chemical Society. 40 (9): 1361–1403. doi:10.1021/ja02242a004.
- Hill, A. V. (1910-01-22). "The possible effects of the aggregation of the molecules of hemoglobin on its dissociation curves". J. Physiol. 40 (Suppl): iv–vii. doi:10.1113/jphysiol.1910.sp001386. S2CID 222195613.
- Weiss, J. N. (1 September 1997). "The Hill equation revisited: uses and misuses". The FASEB Journal. 11 (11): 835–841. doi:10.1096/fasebj.11.11.9285481. ISSN 0892-6638. PMID 9285481. S2CID 827335.
- "Proceedings of the Physiological Society: January 22, 1910". The Journal of Physiology. 40 (suppl): i–vii. 1910. doi:10.1113/jphysiol.1910.sp001386. ISSN 1469-7793. S2CID 222195613.
- Stefan, Melanie I.; Novère, Nicolas Le (27 June 2013). "Cooperative Binding". PLOS Computational Biology. 9 (6): e1003106. Bibcode:2013PLSCB...9E3106S. doi:10.1371/journal.pcbi.1003106. ISSN 1553-7358. PMC 3699289. PMID 23843752.
- Nelson, David L.; Cox, Michael M. (2013). Lehninger principles of biochemistry (6th ed.). New York: W.H. Freeman. pp. 158–162. ISBN 978-1429234146.
- Hamilton, MA; Russo, RC; Thurston, RV (1977). "Trimmed Spearman–Karber method for estimating median lethal concentrations in toxicity bioassays". Environmental Science & Technology. 11 (7): 714–9. Bibcode:1977EnST...11..714H. doi:10.1021/es60130a004.
- Bates, Douglas M.; Watts, Donald G. (1988). Nonlinear Regression Analysis and its Applications. Wiley. p. 365. ISBN 9780471816430.
- Alon, Uri (2007). An Introduction to Systems Biology: Design Principles of Biological Circuits ([Nachdr.] ed.). Boca Raton, FL: Chapman & Hall. ISBN 978-1-58488-642-6.
- Altszyler, E; Ventura, A. C.; Colman-Lerner, A.; Chernomoretz, A. (2017). "Ultrasensitivity in signaling cascades revisited: Linking local and global ultrasensitivity estimations". PLOS ONE. 12 (6): e0180083. arXiv:1608.08007. Bibcode:2017PLoSO..1280083A. doi:10.1371/journal.pone.0180083. PMC 5491127. PMID 28662096.
- Srinivasan, Bharath (2021). "Explicit Treatment of Non‐Michaelis‐Menten and Atypical Kinetics in Early Drug Discovery*". ChemMedChem. 16 (6): 899–918. doi:10.1002/cmdc.202000791. PMID 33231926. S2CID 227157473.
- Srinivasan, Bharath (2021-07-16). "A Guide to the Michaelis‐Menten equation: Steady state and beyond". The FEBS Journal: febs.16124. doi:10.1111/febs.16124. ISSN 1742-464X. PMID 34270860.
- Srinivasan, Bharath (2020-09-27). "Words of advice: teaching enzyme kinetics". The FEBS Journal. 288 (7): 2068–2083. doi:10.1111/febs.15537. ISSN 1742-464X. PMID 32981225.
- Foreman, John (2003). Textbook of Receptor Pharmacology, Second Edition. p. 14. ISBN 9780849310294.
- Ding, S; Sachs, F (1999). "Single Channel Properties of P2X2 Purinoceptors". J. Gen. Physiol. The Rockefeller University Press. 113 (5): 695–720. doi:10.1085/jgp.113.5.695. PMC 2222910. PMID 10228183.
- Chu, Dominique; Zabet, Nicolae Radu; Mitavskiy, Boris (2009-04-07). "Models of transcription factor binding: Sensitivity of activation functions to model assumptions" (PDF). Journal of Theoretical Biology. 257 (3): 419–429. Bibcode:2009JThBi.257..419C. doi:10.1016/j.jtbi.2008.11.026. PMID 19121637.
- Monod, Jacque; Wyman, Jeffries; Changeux, Jean-Pierre (1 May 1965). "On the nature of allosteric transitions: A plausible model". Journal of Molecular Biology. 12 (1): 88–118. doi:10.1016/S0022-2836(65)80285-6. PMID 14343300.
- Kholodenko, Boris N.; et al. (1997). "Quantification of information transfer via cellular signal transduction pathways". FEBS Letters. 414 (2): 430–434. doi:10.1016/S0014-5793(97)01018-1. PMID 9315734. S2CID 19466336.
Further reading
- Dorland's Illustrated Medical Dictionary
- Coval, ML (December 1970). "Analysis of Hill interaction coefficients and the invalidity of the Kwon and Brown equation". J. Biol. Chem. 245 (23): 6335–6. doi:10.1016/S0021-9258(18)62614-6. PMID 5484812.
- d'A Heck, Henry (1971). "Statistical theory of cooperative binding to proteins. Hill equation and the binding potential". J. Am. Chem. Soc. 93 (1): 23–29. doi:10.1021/ja00730a004. PMID 5538860.
- Atkins, Gordon L. (1973). "A simple digital-computer program for estimating the parameter of the Hill Equation". Eur. J. Biochem. 33 (1): 175–180. doi:10.1111/j.1432-1033.1973.tb02667.x. PMID 4691349.
- Endrenyi, Laszlo; Kwong, F. H. F.; Fajszi, Csaba (1975). "Evaluation of Hill slopes and Hill coefficients when the saturation binding or velocity is not known". Eur. J. Biochem. 51 (2): 317–328. doi:10.1111/j.1432-1033.1975.tb03931.x. PMID 1149734.
- Voet, Donald; Voet, Judith G. (2004). Biochemistry.
- Weiss, J. N. (1997). "The Hill equation revisited: uses and misuses". FASEB Journal. 11 (11): 835–841. doi:10.1096/fasebj.11.11.9285481. PMID 9285481. S2CID 827335.
- Kurganov, B. I.; Lobanov, A. V. (2001). "Criterion for Hill equation validity for description of biosensor calibration curves". Anal. Chim. Acta. 427 (1): 11–19. doi:10.1016/S0003-2670(00)01167-3.
- Goutelle, Sylvain; Maurin, Michel; Rougier, Florent; Barbaut, Xavier; Bourguignon, Laurent; Ducher, Michel; Maire, Pascal (2008). "The Hill equation: a review of its capabilities in pharmacological modelling". Fundamental & Clinical Pharmacology. 22 (6): 633–648. doi:10.1111/j.1472-8206.2008.00633.x. PMID 19049668. S2CID 4979109.
- Gesztelyi R; Zsuga J; Kemeny-Beke A; Varga B; Juhasz B; Tosaki A (2012). "The Hill equation and the origin of quantitative pharmacology". Archive for History of Exact Sciences. 66 (4): 427–38. doi:10.1007/s00407-012-0098-5. S2CID 122929930.
- Colquhoun D (2006). "The quantitative analysis of drug-receptor interactions: a short history". Trends Pharmacol Sci. 27 (3): 149–57. doi:10.1016/j.tips.2006.01.008. PMID 16483674.
- Rang HP (2006). "The receptor concept: pharmacology's big idea". Br J Pharmacol. 147: S9–16. doi:10.1038/sj.bjp.0706457. PMC 1760743. PMID 16402126.