Kallidin
Kallidin is a bioactive kinin formed in response to injury from kininogen precursors through the action of kallikreins.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
L-Lysyl-L-arginyl-L-prolyl-L-prolyl-glycyl-L-phenylalanyl-L-seryl-L-prolyl-L-phenylalanyl-L-arginine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.853 |
| MeSH | Kallidin |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C56H85N17O12 |
| Molar mass | 1188.403 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
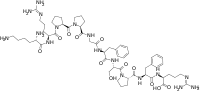
Kallidin is a decapeptide whose sequence is H-Lys-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH. It can be converted to bradykinin by the aminopeptidase enzyme.
It can be a substrate for carboxypeptidase M and N.[1]
Kallidin is identical to bradykinin with an additional lysine residue added at the N-terminal end and signals through the bradykinin receptor.
References
- Stefan Offermanns; Walter Rosenthal (2008). Encyclopedia of Molecular Pharmacology. Springer. pp. 673–. ISBN 978-3-540-38916-3. Retrieved 11 December 2010.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.