Lodoxamide
Lodoxamide is an antiallergic pharmaceutical drug. It is marketed under the tradename Alomide in the UK. Like cromoglicic acid it acts as a mast cell stabilizer.[1] In 2014 lodoxamide and bufrolin were found to be potent agonists at the G protein-coupled receptor 35, an orphan receptor believed to play a role in inflammatory processes, pain and the development of stomach cancer.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
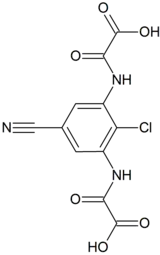
N,N′-(2-Chloro-5-cyano-1,3-phenylene)dioxamic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C11H6ClN3O6 |
| Molar mass | 311.63 g·mol−1 |
| Pharmacology | |
| S01GX05 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
- Nedocromil
- Zaprinast
- Amlexanox
- Pemirolast
- Pamoic acid
- Kynurenic acid
- CXCL17
References
- Avunduk, A. M.; Avunduk, M. C.; Kapicioglu, Z.; Akyol, N.; Tavli, L. (2000). "Mechanisms and comparison of anti-allergic efficacy of topical lodoxamide and cromolyn sodium treatment in vernal keratoconjunctivitis". Ophthalmology. 107 (7): 1333–1337. doi:10.1016/S0161-6420(00)00089-0. PMID 10890862.
- MacKenzie, AE; Caltabiano, G; Kent, TC; Jenkins, L; McCallum, JE; Hudson, BD; Nicklin, SA; Fawcett, L; Markwick, R; Charlton, SJ; Milligan, G (2014). "The antiallergic mast cell stabilizers lodoxamide and bufrolin as the first high and equipotent agonists of human and rat GPR35". Molecular Pharmacology. 85 (1): 91–104. doi:10.1124/mol.113.089482. ISSN 0026-895X. PMC 3868900. PMID 24113750.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.