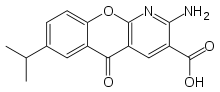
Amlexanox
Amlexanox (trade name Aphthasol) is an anti-inflammatory antiallergic immunomodulator used to treat recurrent aphthous ulcers (canker sores), and (in Japan) several inflammatory conditions. This drug has been discontinued in the U.S.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Aphthasol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601017 |
| Routes of administration | Topical |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 3.5 hours |
| Excretion | Renal (17%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.230.878 |
| Chemical and physical data | |
| Formula | C16H14N2O4 |
| Molar mass | 298.298 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Medical uses
Amlexanox is the active ingredient in a common topical treatment for recurrent aphthous ulcers of the mouth (canker sores),[2] reducing both healing time[3] and pain.[4] Amlexanox 5% paste is well tolerated,[5] and is typically applied four times per day directly on the ulcers.[3] A 2011 review found it to be the most effective treatment of the eight treatments investigated for recurrent canker sores.[6] It is also used to treat ulcers associated with Behçet disease.[7]
In Japan, it is used to treat bronchial asthma, allergic rhinitis and conjunctivitis.[8]
Contraindications
The drug is contraindicated in those with known allergies to it.[3]
Adverse effects
Amlexanox may cause a slightly painful stinging or burning sensation, nausea or diarrhea.[3]
Mechanism of action
Its mechanism of action is not well-determined, but it might inhibit inflammation by inhibiting the release of histamine and leukotrienes.[8] It has been shown to selectively inhibit TBK1 and IKK-ε, producing reversible weight loss and improved insulin sensitivity, reduced inflammation and attenuated hepatic steatosis without affecting food intake in obese mice.[9] It produced a statistically significant reduction in glycated hemoglobin and fructosamine in obese patients with type 2 diabetes and nonalcoholic fatty liver disease[10]
Chemistry
The chemical itself is an odorless, white to yellowish-white powder.[8]
The 5% preparation for patient use is an adherent beige paste,[3][8] and it is also available in some countries as a tablet that adheres to the ulcer in the mouth.[4]
Pharmacokinetics
Amlexanox applied to an aphthous ulcer is largely absorbed through the gastrointestinal tract; an insignificant amount enters the bloodstream through the ulcer itself. After a single 100 mg dose, mean maximum serum concentration occurs 2.4 +/- 0.9 hours after application, with a half-life of elimination (through urine) of 3.5 +/- 1.1 hours. With multiple daily applications (four doses per day), steady state serum levels occur after one week, with no accumulation occurring after four weeks.[8]
History
The patent for its use as a treatment for aphthous ulcers was issued in November 1994 to inventors Kakubhai R. Vora, Atul Khandwala and Charles G. Smith, and assigned to Chemex Pharmaceuticals, Inc.[11]
Society and culture
Economics
A 2011 review found a one-week supply of amlexanox 5% paste to cost $30.[6]
Research
A review found that, as of July 2011, robust studies investigating its effectiveness alongside other canker sore treatments were still needed.[12]
Because it is an inhibitor of the protein kinases TBK1 and IKK-ε,[9] which are implicated in the etiology of type II diabetes and obesity,[13] amlexanox may be a candidate for human clinical trials testing in relation to these diseases.[9]
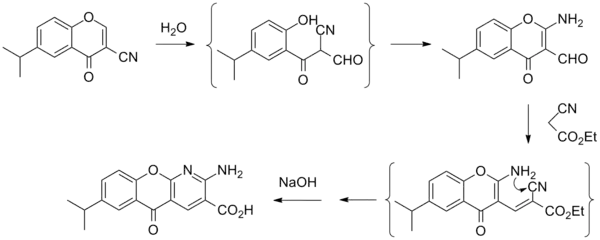
Synthesis
References
- "Amlexanox (Aphthasol®)". Archived from the original on 20 November 2013. Retrieved 20 November 2013.
- Gonsalves WC, Chi AC, Neville BW (February 2007). "Common oral lesions: Part I. Superficial mucosal lesions". American Family Physician. 75 (4): 501–507. PMID 17323710.
- "Amlexanox". MedlinePlus. U.S. National Library of Medicine. February 2009. Retrieved 12 February 2013.
- Plewa MC (March 2012). "Pediatric Aphthous Ulcers Treatment & Management". Medscape Reference. Medscape. Retrieved 14 February 2013.
- "Amlexanox". PubChem. U.S. National Library of Medicine. Retrieved 12 February 2013.
- Bailey J, McCarthy C, Smith RF (October 2011). "Clinical inquiry. What is the most effective way to treat recurrent canker sores?". The Journal of Family Practice. 60 (10): 621–632. PMID 21977491.
- Yousefi M, Ferringer T, Lee S, Bang D (July 2012). "Dermatologic Aspects of Behcet Disease Treatment & Management". Medscape Reference. Medscape. Retrieved 14 February 2013.
- Bell J (2005). "Amlexanox for the treatment of recurrent aphthous ulcers". Clinical Drug Investigation. 25 (9): 555–566. doi:10.2165/00044011-200525090-00001. PMID 17532700.
- Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, et al. (March 2013). "An inhibitor of the protein kinases TBK1 and IKK-ɛ improves obesity-related metabolic dysfunctions in mice". Nature Medicine. 19 (3): 313–321. doi:10.1038/nm.3082. PMC 3594079. PMID 23396211.
- Oral EA, Reilly SM, Gomez AV, Meral R, Butz L, Ajluni N, et al. (July 2017). "Inhibition of IKKɛ and TBK1 Improves Glucose Control in a Subset of Patients with Type 2 Diabetes". Cell Metabolism. 26 (1): 157–170.e7. doi:10.1016/j.cmet.2017.06.006. PMC 5663294. PMID 28683283.
- US patent 5362737, Vora KR, Khandwala A, Smith CG, "Methods of treating aphthous ulcers and other mucocutaneous disorders with amlexanox", published 1994-11-08, assigned to Chemex Pharmaceuticals, Inc.
- Kuteyi T, Okwundu CI (January 2012). Kuteyi T (ed.). "Topical treatments for HIV-related oral ulcers". The Cochrane Database of Systematic Reviews. 1: CD007975. doi:10.1002/14651858.CD007975.pub2. PMID 22258979.
- Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, et al. (September 2009). "The protein kinase IKKepsilon regulates energy balance in obese mice". Cell. 138 (5): 961–975. doi:10.1016/j.cell.2009.06.046. PMC 2756060. PMID 19737522.
- Nohara A, Ishiguro T, Ukawa K, Sugihara H, Maki Y, Sanno Y (May 1985). "Studies on antianaphylactic agents. 7. Synthesis of antiallergic 5-oxo-5H-[1]benzopyrano[2,3-b]pyridines". Journal of Medicinal Chemistry. 28 (5): 559–568. doi:10.1021/jm50001a005. PMID 3989816.