Doxycycline
Doxycycline is a broad-spectrum tetracycline class antibiotic used in the treatment of infections caused by bacteria and certain parasites.[1] It is used to treat bacterial pneumonia, acne, chlamydia infections, Lyme disease, cholera, typhus, and syphilis.[1] It is also used to prevent malaria in combination with quinine.[1] Doxycycline may be taken by mouth or by injection into a vein.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌdɒksɪˈsaɪkliːn/ DOKS-iss-EYE-kleen |
| Trade names | Doxy, Doryx, Vibramycin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682063 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous[1] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Protein binding | 80–90% |
| Metabolism | Negligible |
| Elimination half-life | 10–22 hours |
| Excretion | Mainly feces, 40% urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.429 |
| Chemical and physical data | |
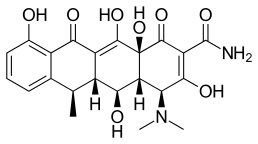
| Formula | C22H24N2O8 |
| Molar mass | 444.440 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Common side effects include diarrhea, nausea, vomiting, abdominal pain, and an increased risk of sunburn.[1] Use during pregnancy is not recommended.[1] Like other agents of the tetracycline class, it either slows or kills bacteria by inhibiting protein production.[1][2] It kills malaria by targeting a plastid organelle, the apicoplast.[3][4]
Doxycycline was patented in 1957 and came into commercial use in 1967.[5][6] It is on the World Health Organization's List of Essential Medicines.[7] Doxycycline is available as a generic medicine.[1][8] In 2020, it was the 79th most commonly prescribed medication in the United States, with more than 9 million prescriptions.[9][10]
Medical use


In addition to the general indications for all members of the tetracycline antibiotics group, doxycycline is frequently used to treat Lyme disease, chronic prostatitis, sinusitis, pelvic inflammatory disease,[11][12] acne, rosacea,[13][14] and rickettsial infections.[15]
In Canada, in 2004, doxycycline was considered a first-line treatment for chlamydia and non-gonococcal urethritis and with cefixime for uncomplicated gonorrhea.[16]
Antibacterial
Moraxella catarrhalis, Brucella melitensis, Chlamydia pneumoniae, and Mycoplasma pneumoniae are generally susceptible to doxycycline, while some Haemophilus spp., Mycoplasma hominis, and Pseudomonas aeruginosa have developed resistance to varying degrees.[17]
It is used in the treatment and prophylaxis of anthrax and Leptospirosis.[18] It is also effective against Yersinia pestis (the infectious agent of bubonic plague), and is prescribed for the treatment of Lyme disease,[19][20][21][22] ehrlichiosis,[23][24] and Rocky Mountain spotted fever.[25]
Doxycycline is indicated for treatment of:[25][26]
- Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever,[27] rickettsialpox, and tick fevers caused by Rickettsia
- Respiratory tract infections caused by Mycoplasma pneumoniae[28]
- Lymphogranuloma venereum, trachoma, inclusion conjunctivitis, and uncomplicated urethral, endocervical, or rectal infections in adults caused by Chlamydia trachomatis
- Psittacosis
- Nongonococcal urethritis caused by Ureaplasma urealyticum
- Relapsing fever due to Borrelia recurrentis
- Chancroid caused by Haemophilus ducreyi
- Plague due to Yersinia pestis
- Tularemia
- Cholera
- Campylobacter fetus infections
- Brucellosis caused by Brucella species (in conjunction with streptomycin)
- Bartonellosis
- Granuloma inguinale (Klebsiella species)
- Lyme disease[29]
When bacteriologic testing indicates appropriate susceptibility to the drug, doxycycline may be used to treat these infections caused by Gram-negative bacteria:[25][26]
- Escherichia coli infections
- Enterobacter aerogenes (formerly Aerobacter aerogenes) infections
- Shigella species infections
- Acinetobacter species (formerly Mima species and Herellea species) infections
- Respiratory tract infections caused by Haemophilus influenzae
- Respiratory tract and urinary tract infections caused by Klebsiella species
Some Gram-positive bacteria have developed resistance to doxycycline. Up to 44% of Streptococcus pyogenes and up to 74% of S. faecalis specimens have developed resistance to the tetracycline group of antibiotics. Up to 57% of P. acnes strains developed resistance to doxycycline.[30] When bacteriologic testing indicates appropriate susceptibility to the drug, doxycycline may be used to treat these infections caused by Gram-positive bacteria:[25][26]
- Upper respiratory infections caused by Streptococcus pneumoniae (formerly Diplococcus pneumoniae)
- Skin and soft tissue infections caused by Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus infections
- Anthrax caused by Bacillus anthracis infection
When penicillin is contraindicated, doxycycline can be used to treat:[25][26]
- Syphilis caused by Treponema pallidum
- Yaws caused by Treponema pertenue
- Listeriosis due to Listeria monocytogenes
- Vincent's infection caused by Fusobacterium fusiforme
- Actinomycosis caused by Actinomyces israelii
- Infections caused by Clostridium species
Doxycycline may also be used as adjunctive therapy for severe acne.[25][26]
The first-line treatment for brucellosis is a combination of doxycycline and streptomycin and the second-line is a combination of doxycycline and rifampicin (rifampin).[31]
Antimalarial
Doxycycline is active against the erythrocytic stages of Plasmodium falciparum but not against the gametocytes of P. falciparum.[32] It is used to prevent malaria.[33] It is not recommended alone for initial treatment of malaria, even when the parasite is doxycycline-sensitive, because the antimalarial effect of doxycycline is delayed.[34]
The World Health Organization (WHO) guidelines state that the combination of doxycycline with either artesunate or quinine may be used for the treatment of uncomplicated malaria due to P. falciparum or following intravenous treatment of severe malaria.[35]
Antihelminthic
Doxycycline kills the symbiotic Wolbachia bacteria in the reproductive tracts of parasitic filarial nematodes, making the nematodes sterile, and thus reducing transmission of diseases such as onchocerciasis and elephantiasis.[36] Field trials in 2005 showed an eight-week course of doxycycline almost eliminates the release of microfilariae.[37]
Spectrum of susceptibility
Doxycycline has been used successfully to treat sexually transmitted, respiratory, and ophthalmic infections. Representative pathogenic genera include Chlamydia, Streptococcus, Ureaplasma, Mycoplasma, and others. The following represents MIC susceptibility data for a few medically significant microorganisms.[38]
- Chlamydia psittaci: 0.03 μg/mL
- Mycoplasma pneumoniae: 0.016 μg/mL—2 μg/mL
- Streptococcus pneumoniae: 0.06 μg/mL—32 μg/mL
Sclerotherapy
Doxycycline is also used for sclerotherapy in slow-flow vascular malformations, namely venous and lymphatic malformations, as well as post-operative lymphoceles.[39]
Others
Subantimicrobial-dose doxycycline (SDD) is widely used as an adjunctive treatment to scaling and root planing for periodontitis. Significant differences were observed for all investigated clinical parameters of periodontitis in favor of the scaling and root planing + SDD group where SDD dosage regimens is 20 mg twice daily for three months in a meta-analysis published in 2011.[40]
Contraindications
Pregnancy and lactation
Doxycycline is categorized by the FDA as a class D drug in pregnancy. Doxycycline crosses into breastmilk.[41] Other tetracycline antibiotics are contraindicated in pregnancy and up to eight years of age, due to the potential for disrupting bone and tooth development.[42] They include a class warning about staining of teeth and decreased development of dental enamel in children exposed to tetracyclines in utero, during breastfeeding or during young childhood.[43] However, the FDA has acknowledged that the actual risk of dental staining of primary teeth is undetermined for doxycycline specifically. The best available evidence indicates that doxycycline has little or no effect on hypoplasia of dental enamel or on staining of teeth and the CDC recommends the use of doxycycline for treatment of Q fever and also for tick-borne rickettsial diseases in young children and others advocate for its use in malaria.[44]
Other
Other contraindications are severe liver disease and concomitant use of isotretinoin or other retinoids, as both tetracyclines and retinoids can cause intracranial hypertension (increased pressure around the brain) in rare cases.[45]
Adverse effects
Adverse effects are similar to those of other members of the tetracycline antibiotic group. Doxycycline can cause gastrointestinal upset.[46][47] Oral doxycycline can cause pill esophagitis, particularly when it is swallowed without adequate fluid, or by persons with difficulty swallowing or impaired mobility.[48] Doxycycline is less likely than other antibiotic drugs to cause Clostridium difficile colitis.[49]
An erythematous rash in sun-exposed parts of the body has been reported to occur in 7.3–21.2% of persons taking doxycycline for malaria prophylaxis. One study examined the tolerability of various malaria prophylactic regimens and found doxycycline did not cause a significantly higher percentage of all skin events (photosensitivity not specified) when compared with other antimalarials. The rash resolves upon discontinuation of the drug.[50]
Unlike some other members of the tetracycline group, it may be used in those with renal impairment.[51]
Doxycycline use has been associated with increased risk of inflammatory bowel disease.[52] In one large retrospective study, patients who were prescribed doxycycline for their acne had a 2.25-fold greater risk of developing Crohn's disease.[53]
Interactions
The combination of doxycycline with dairy, antacids, calcium supplements, iron products, laxatives containing magnesium, or bile acid sequestrants is not inherently dangerous, but any of these foods and supplements may decrease doxycycline's effectiveness.[45][54]
Breakfast was observed to reduce doxycycline absorption significantly. Absorption of tetracycline occurs in the stomach and the upper small intestine. Absorption of tetracyclines has been reported to be impaired by milk products, aluminum hydroxide gels, sodium bicarbonate, calcium and magnesium salts, laxatives containing magnesium and iron preparations. The mechanisms responsible for decreased absorption appear to be chelation and an increase in gastric pH. ... In view of these results, it is advisable to instruct the patients to take doxycycline on an empty stomach.[55]
Previously, doxycycline was believed to impair the effectiveness of many types of hormonal contraception due to CYP450 induction. Research has shown no significant loss of effectiveness in oral contraceptives while using most tetracycline antibiotics (including doxycycline), although many physicians still recommend the use of barrier contraception for people taking the drug to prevent unwanted pregnancy.[56][57][58]
Pharmacology
Doxycycline, like other tetracycline antibiotics, is bacteriostatic. It works by preventing bacteria from reproducing through the inhibition of protein synthesis.[59]
Doxycycline is highly lipophilic, so it can easily enter cells, meaning the drug is easily absorbed after oral administration and has a large volume of distribution. It can also be re-absorbed in the renal tubules and gastrointestinal tract due to its high lipophillicity, giving it a long elimination half life, and it is also prevented from accumulating in the kidneys of patients with kidney failure due to the compensatory excretion in faeces.[47][60] Doxycycline–metal ion complexes are unstable at acid pH, therefore more doxycycline enters the duodenum for absorption than the earlier tetracycline compounds. In addition, food has less effect on absorption than on absorption of earlier drugs with doxycycline serum concentrations being reduced by about 20% by test meals compared with 50% for tetracycline.[61]
Mechanism of action
Doxycycline is a broad-spectrum bacteriostatic antibiotic. It inhibits the synthesis of bacterial proteins by binding to the 30S ribosomal subunit, which is only found in bacteria.[46][60] This prevents the binding of transfer RNA to messenger RNA at the ribosomal subunit meaning amino acids cannot be added to polypeptide chains and new proteins cannot be made. This stops bacterial growth giving the immune system time to kill and remove the bacteria.[62]
Pharmacokinetics
The substance is almost completely absorbed from the upper part of the small intestine. It reaches highest concentrations in the blood plasma after one to two hours and has a high plasma protein binding rate of about 80–90%. Doxycycline penetrates into almost all tissues and body fluids. Very high concentrations are found in the gallbladder, liver, kidneys, lung, breast milk, bone and genitals; low ones in saliva, aqueous humour, cerebrospinal fluid (CSF), and especially in inflamed meninges.[45][63][64] By comparison, the tetracycline antibiotic minocycline penetrates significantly better into the CSF and meninges.[65]
Doxycycline metabolism is negligible. It is actively excreted into the gut (in part via the gallbladder, in part directly from blood vessels), where some of it is inactivated by forming chelates. About 40% are eliminated via the kidneys, much less in people with end-stage kidney disease. The biological half-life is 18 to 22 hours (16±6 hours according to another source[63]) in healthy people, slightly longer in those with end-stage kidney disease, and significantly longer in those with liver disease.[45][63][64]
Chemistry
Expired tetracyclines or tetracyclines allowed to stand at a pH less than 2 are reported to be nephrotoxic due to the formation of a degradation product, anhydro-4-epitetracycline[66][67] causing Fanconi syndrome.[68] In the case of doxycycline, the absence of a hydroxyl group in C-6 prevents the formation of the nephrotoxic compound.[67] Nevertheless, tetracyclines and doxycycline itself have to be taken with caution in patients with kidney injury, as they can worsen azotemia due to catabolic effects.[68]
Chemical properties
Doxycycline, doxycycline monohydrate and doxycycline hyclate are yellow, crystalline powders with a bitter taste. The latter smells faintly of ethanol; a 1% aqueous solution has a pH of 2–3; and the specific rotation is −110° cm³/dm·g in 0.01 N methanolic hydrochloric acid.[63]
| Solubility in | Doxycycline | Doxycycline monohydrate | Doxycycline Hyclate |
|---|---|---|---|
| Water | very slightly | very slightly | freely |
| Ethanol | very slightly | very slightly | sparingly |
| Aqueous acids | freely | freely | |
| Alkali hydroxyde solutions | freely | freely | |
| Chloroform | very slightly | practically insoluble | practically insoluble |
| Diethyl ether | insoluble | practically insoluble | practically insoluble |
History
After penicillin revolutionized the treatment of bacterial infections in WWII, many chemical companies moved into the field of discovering antibiotics by bioprospecting. American Cyanamid was one of these, and in the late 1940s chemists there discovered chlortetracycline, the first member of the tetracycline class of antibiotics.[2] Shortly thereafter, scientists at Pfizer discovered terramycin and it was brought to market. Both compounds, like penicillin, were natural products and it was commonly believed that nature had perfected them, and further chemical changes could only degrade their effectiveness. Scientists at Pfizer led by Lloyd Conover modified these compounds, which led to the invention of tetracycline itself, the first semi-synthetic antibiotic. Charlie Stephens' group at Pfizer worked on further analogs and created one with greatly improved stability and pharmacological efficacy: doxycycline. It was clinically developed in the early 1960s and approved by the FDA in 1967.[2]
As its patent grew near to expiring in the early 1970s, the patent became the subject of lawsuit between Pfizer and International Rectifier[69] that was not resolved until 1983; at the time it was the largest litigated patent case in US history.[70] Instead of a cash payment for infringement, Pfizer took the veterinary and feed-additive businesses of International Rectifier's subsidiary, Rachelle Laboratories.[70]
In January 2013, the FDA reported shortages of some, but not all, forms of doxycycline "caused by increased demand and manufacturing issues".[71] Companies involved included an unnamed major generics manufacturer that ceased production in February 2013, Teva (which ceased production in May 2013), Mylan, Actavis, and Hikma Pharmaceuticals.[72][73] The shortage came at a particularly bad time, since there were also shortages of an alternative antibiotic, tetracycline, at the same time.[74] The market price for doxycycline dramatically increased in the United States in 2013 and early 2014 (from $20 to over $1800 for a bottle of 500 tablets),[75][76][77] before decreasing again.[78][79]
Society and culture
Doxycycline is available worldwide under many brand names.[80] Doxycycline is available as a generic medicine.[1][8]
Research
Research areas have included:
- Macular degeneration[81]
- Rheumatoid arthritis instead of minocycline (both of which have demonstrated modest efficacy for this disease)[82]

Research reagent
Doxycycline and other members of the tetracycline class of antibiotics are often used as research reagents in in vitro and in vivo biomedical research experiments involving bacteria as well in experiments in eukaryotic cells and organisms with inducible protein expression systems using tetracycline-controlled transcriptional activation. The mechanism of action for the antibacterial effect of tetracyclines relies on disrupting protein translation in bacteria, thereby damaging the ability of microbes to grow and repair; however protein translation is also disrupted in eukaryotic mitochondria impairing metabolism and leading to effects that can confound experimental results.[83][84] Doxycycline is also used in "tet-on" (gene expression activated by doxycycline) and "tet-off" (gene expression inactivated by doxycycline) tetracycline-controlled transcriptional activation to regulate transgene expression in organisms and cell cultures.[85] Doxycycline is more stable than tetracycline for this purpose.[85] At subantimicrobial doses, doxycycline is an inhibitor of matrix metalloproteases, and has been used in various experimental systems for this purpose, such as for recalcitrant recurrent corneal erosions.[86]
COVID-19
After a large-scale trial showed no benefit of using doxycycline in treating COVID‑19, the UK's National Institute for Health and Care Excellence (NICE) updated its guidance to not recommend the medication for the treatment of COVID‑19.[87][88]
References
- "Doxycycline calcium". The American Society of Health-System Pharmacists. Archived from the original on 23 September 2015. Retrieved 18 August 2015.
- Nelson ML, Levy SB (December 2011). "The history of the tetracyclines". Annals of the New York Academy of Sciences. 1241 (1): 17–32. Bibcode:2011NYASA1241...17N. doi:10.1111/j.1749-6632.2011.06354.x. PMID 22191524. S2CID 34647314.
- McFadden GI (March 2014). "Apicoplast". Current Biology. 24 (7): R262-3. doi:10.1016/j.cub.2014.01.024. PMID 24698369.
- Schlagenhauf-Lawlor P (2008). Travelers' Malaria. PMPH-USA. p. 148. ISBN 9781550093360.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 489. ISBN 9783527607495.
- Corey EJ (2013). Drug discovery practices, processes, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 406. ISBN 9781118354469.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Hamilton RJ (2011). Tarascon pharmacopoeia (12th ed.). Sudbury, MA: Jones & Bartlett Learning. p. 79. ISBN 9781449600679.
- "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- "Doxycycline - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- Sweet RL, Schachter J, Landers DV, Ohm-Smith M, Robbie MO (March 1988). "Treatment of hospitalized patients with acute pelvic inflammatory disease: comparison of cefotetan plus doxycycline and cefoxitin plus doxycycline". American Journal of Obstetrics and Gynecology. 158 (3 Pt 2): 736–41. doi:10.1016/S0002-9378(16)44537-0. PMID 3162653.
- Gjønnaess H, Holten E (1978). "Doxycycline (Vibramycin) in pelvic inflammatory disease". Acta Obstetricia et Gynecologica Scandinavica. 57 (2): 137–9. doi:10.3109/00016347809155893. PMID 345730. S2CID 28328073.
- Määttä M, Kari O, Tervahartiala T, Peltonen S, Kari M, Saari M, Sorsa T (August 2006). "Tear fluid levels of MMP-8 are elevated in ocular rosacea--treatment effect of oral doxycycline". Graefe's Archive for Clinical and Experimental Ophthalmology = Albrecht von Graefes Archiv für Klinische und Experimentelle Ophthalmologie. 244 (8): 957–62. doi:10.1007/s00417-005-0212-3. PMID 16411105. S2CID 20540747.
- Quarterman MJ, Johnson DW, Abele DC, Lesher JL, Hull DS, Davis LS (January 1997). "Ocular rosacea. Signs, symptoms, and tear studies before and after treatment with doxycycline". Archives of Dermatology. 133 (1): 49–54. doi:10.1001/archderm.133.1.49. PMID 9006372.
- Walker DH, Paddock CD, Dumler JS (November 2008). "Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections". The Medical Clinics of North America. 92 (6): 1345–61, x. doi:10.1016/j.mcna.2008.06.002. PMID 19061755.
- Michael L. Rekart (December 2014). "Doxycycline: "New" treatment of choice for genital chlamydia infections". Archived from the original on 2 February 2017.
- "Doxycycline spectrum of bacterial susceptibility and Resistance" (PDF). Archived from the original (PDF) on 1 February 2014. Retrieved 4 May 2012.
- Stoddard RA, Galloway RL, Guerra MA (10 July 2015). "Leptospirosis". Yellow Book. Atlanta, GA: Centers for Disease Control and Prevention. Archived from the original on 9 April 2017. Retrieved 16 April 2017.
- Nadelman RB, Luger SW, Frank E, Wisniewski M, Collins JJ, Wormser GP (August 1992). "Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease". Annals of Internal Medicine. 117 (4): 273–80. doi:10.7326/0003-4819-117-4-273. PMID 1637021.
- Luger SW, Paparone P, Wormser GP, Nadelman RB, Grunwaldt E, Gomez G, et al. (March 1995). "Comparison of cefuroxime axetil and doxycycline in treatment of patients with early Lyme disease associated with erythema migrans". Antimicrobial Agents and Chemotherapy. 39 (3): 661–7. doi:10.1128/AAC.39.3.661. PMC 162601. PMID 7793869.
- Nadelman RB, Nowakowski J, Fish D, Falco RC, Freeman K, McKenna D, et al. (July 2001). "Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite". The New England Journal of Medicine. 345 (2): 79–84. doi:10.1056/NEJM200107123450201. PMID 11450675.
- Karlsson M, Hammers-Berggren S, Lindquist L, Stiernstedt G, Svenungsson B (July 1994). "Comparison of intravenous penicillin G and oral doxycycline for treatment of Lyme neuroborreliosis". Neurology. 44 (7): 1203–7. doi:10.1212/WNL.44.7.1203. PMID 8035916. S2CID 38661885.
- Weinstein RS (November 1996). "Human ehrlichiosis". American Family Physician. 54 (6): 1971–6. PMID 8900357.
- Karlsson U, Bjöersdorff A, Massung RF, Christensson B (2001). "Human granulocytic ehrlichiosis--a clinical case in Scandinavia". Scandinavian Journal of Infectious Diseases. 33 (1): 73–4. doi:10.1080/003655401750064130. PMID 11234985. S2CID 218880245.
- U.S. Food and Drug Administration. 14 December 2012. Doxycycline, ANDA no. 065055 Label. Archived 19 April 2014 at the Wayback Machine
- U.S. Food and Drug Administration. 16 July 2008.Doxycycline, ANDA no. 065454 Label Archived 19 October 2013 at the Wayback Machine
- Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, et al. (March 2013). "Diagnosis and management of Q fever--United States, 2013: recommendations from CDC and the Q Fever Working Group". MMWR. Recommendations and Reports. 62 (RR-03): 1–30. PMID 23535757. Archived from the original on 19 April 2014.
- Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, et al. (December 2012). "Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children". Clinical Infectious Diseases. 55 (12): 1642–9. doi:10.1093/cid/cis784. PMID 22972867.
- "Lyme disease. Treatment". 21 December 2018. Archived from the original on 10 June 2016.
- Dreno B, Thiboutot D, Gollnick H, Bettoli V, Kang S, Leyden JJ, et al. (2014). "Antibiotic stewardship in dermatology: limiting antibiotic use in acne". European Journal of Dermatology. 24 (3): 330–4. doi:10.1684/ejd.2014.2309. PMID 24721547. S2CID 28700961.
- William Cameron (2012). "Comparison of doxycycline–streptomycin, doxycycline–rifampin, and ofloxacin–rifampin in the treatment of brucellosis: a randomized clinical trial". International Journal of Infectious Diseases. 16 (4): e247–e251. doi:10.1016/j.ijid.2011.12.003. PMID 22296864. Retrieved 23 August 2014.
- "Doryx- doxycycline hyclate tablet, delayed release". DailyMed. 23 October 2020. Retrieved 5 March 2022.
- "Malaria - Chapter 3 - 2018 Yellow Book | Travelers' Health | CDC". CDC. Retrieved 4 December 2018.
- Dahl EL, Shock JL, Shenai BR, Gut J, DeRisi JL, Rosenthal PJ (September 2006). "Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum". Antimicrobial Agents and Chemotherapy. 50 (9): 3124–31. doi:10.1128/AAC.00394-06. PMC 1563505. PMID 16940111.
- Guidelines for the treatment of malaria. Geneva: World Health Organization. 2015. p. 246. ISBN 978-92-4-154912-7.
- Hoerauf A, Mand S, Fischer K, Kruppa T, Marfo-Debrekyei Y, Debrah AY, et al. (November 2003). "Doxycycline as a novel strategy against bancroftian filariasis-depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production". Medical Microbiology and Immunology. 192 (4): 211–6. doi:10.1007/s00430-002-0174-6. PMID 12684759. S2CID 23349595.
- Taylor MJ, Makunde WH, McGarry HF, Turner JD, Mand S, Hoerauf A (2005). "Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: a double-blind, randomised placebo-controlled trial". Lancet. 365 (9477): 2116–21. doi:10.1016/S0140-6736(05)66591-9. PMID 15964448. S2CID 21382828.
- "Doxycycline hyclate Susceptibility and Minimum Inhibitory Concentration (MIC) Data" (PDF). toku-e.com. Retrieved 16 April 2017.
- Kaufman, John A.; Lee, Michael J. (22 June 2013). Vascular and interventional radiology (2nd ed.). Philadelphia, PA. ISBN 978-0-323-07672-2. OCLC 853455295.
- Sgolastra F, Petrucci A, Gatto R, Giannoni M, Monaco A (November 2011). "Long-term efficacy of subantimicrobial-dose doxycycline as an adjunctive treatment to scaling and root planing: a systematic review and meta-analysis". Journal of Periodontology. 82 (11): 1570–81. doi:10.1902/jop.2011.110026. PMID 21417590.
- Chung AM, Reed MD, Blumer JL (2002). "Antibiotics and breast-feeding: a critical review of the literature". Paediatric Drugs. 4 (12): 817–37. doi:10.2165/00128072-200204120-00006. PMID 12431134. S2CID 8595370.
- Mylonas I (January 2011). "Antibiotic chemotherapy during pregnancy and lactation period: aspects for consideration". Archives of Gynecology and Obstetrics. 283 (1): 7–18. doi:10.1007/s00404-010-1646-3. PMID 20814687. S2CID 25492353.
- "Bioterrorism and Drug Preparedness - Doxycycline Use by Pregnant and Lactating Women". FDA. 3 November 2018. Retrieved 9 December 2018.
- Gaillard T, Briolant S, Madamet M, Pradines B (April 2017). "The end of a dogma: the safety of doxycycline use in young children for malaria treatment". Malaria Journal. 16 (1): 148. doi:10.1186/s12936-017-1797-9. PMC 5390373. PMID 28407772.
- Haberfeld H, ed. (2020). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Doxycyclin Genericon 200 mg lösliche Tabletten.
- Hitchings A, Lonsdale D, Burrage D, Baker E (2015). Top 100 drugs : clinical pharmacology and practical prescribing. pp. 200–201. ISBN 978-0-7020-5516-4.
- Riond JL, Riviere JE (October 1988). "Pharmacology and toxicology of doxycycline". Veterinary and Human Toxicology. 30 (5): 431–43. PMID 3055652.
- Affolter K, Samowitz W, Boynton K, Kelly ED (August 2017). "Doxycycline-induced gastrointestinal injury". Human Pathology. 66: 212–215. doi:10.1016/j.humpath.2017.02.011. PMID 28286288.
- Hung YP, Lee JC, Lin HJ, Liu HC, Wu YH, Tsai PJ, Ko WC (June 2015). "Doxycycline and Tigecycline: Two Friendly Drugs with a Low Association with Clostridium Difficile Infection". Antibiotics. 4 (2): 216–29. doi:10.3390/antibiotics4020216. PMC 4790331. PMID 27025622.
- Tan KR, Magill AJ, Parise ME, Arguin PM (April 2011). "Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis". The American Journal of Tropical Medicine and Hygiene. 84 (4): 517–31. doi:10.4269/ajtmh.2011.10-0285. PMC 3062442. PMID 21460003.
- Dréno B, Bettoli V, Ochsendorf F, Layton A, Mobacken H, Degreef H (November–December 2004). "European recommendations on the use of oral antibiotics for acne". European Journal of Dermatology. 14 (6): 391–9. PMID 15564203. Archived from the original on 29 May 2007. Retrieved 31 January 2008.
- Lee TW, Russell L, Deng M, Gibson PR (August 2013). "Association of doxycycline use with the development of gastroenteritis, irritable bowel syndrome and inflammatory bowel disease in Australians deployed abroad". Internal Medicine Journal. 43 (8): 919–26. doi:10.1111/imj.12179. PMID 23656210. S2CID 9418654.
- Margolis DJ, Fanelli M, Hoffstad O, Lewis JD (December 2010). "Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease". The American Journal of Gastroenterology. 105 (12): 2610–6. doi:10.1038/ajg.2010.303. PMID 20700115. S2CID 20085592.
- PubMed Health (1 July 2016). "Doxycycline (By mouth)". U.S. National Library of Medicine. Archived from the original on 11 November 2013. Retrieved 16 July 2016.
- Kshirsagar N A, Ankalesaria P S. Effect of food on doxycycline absorption. J Postgrad Med (serial online) 1987 (cited 2016 Jul 16);33:117. Available from: http://www.jpgmonline.com/text.asp?1987/33/3/117/5279 Archived 18 August 2016 at the Wayback Machine
- Archer JS, Archer DF (June 2002). "Oral contraceptive efficacy and antibiotic interaction: a myth debunked". Journal of the American Academy of Dermatology. 46 (6): 917–23. doi:10.1067/mjd.2002.120448. PMID 12063491.
- Dréno B, Bettoli V, Ochsendorf F, Layton A, Mobacken H, Degreef H (November–December 2004). "European recommendations on the use of oral antibiotics for acne" (PDF). European Journal of Dermatology. 14 (6): 391–9. PMID 15564203.
- DeRossi SS, Hersh EV (October 2002). "Antibiotics and oral contraceptives". Dental Clinics of North America. 46 (4): 653–64. CiteSeerX 10.1.1.620.9933. doi:10.1016/S0011-8532(02)00017-4. PMID 12436822.
- Flower, R.; Rang, H. P.; Dale, M. M.; Ritter, J. M.; Henderson, G. (2012). Rang & Dale's Pharmacology. Edinburgh: Churchill Livingstone. ISBN 978-0-7020-3471-8.
- Maaland MG, Papich MG, Turnidge J, Guardabassi L (November 2013). "Pharmacodynamics of doxycycline and tetracycline against Staphylococcus pseudintermedius: proposal of canine-specific breakpoints for doxycycline". Journal of Clinical Microbiology. 51 (11): 3547–54. doi:10.1128/JCM.01498-13. PMC 3889732. PMID 23966509.
- Agwuh KN, MacGowan A (August 2006). "Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines". The Journal of Antimicrobial Chemotherapy. 58 (2): 256–65. doi:10.1093/jac/dkl224. PMID 16816396.
- "Doxycycline". www.drugbank.ca. Retrieved 23 January 2019.
- Dinnendahl, V; Fricke, U, eds. (2010). Arzneistoff-Profile (in German). Vol. 4 (24 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. Doxycyclin. ISBN 978-3-7741-9846-3.
- Doxycycline Professional Drug Facts. Accessed 5 August 2020.
- Haberfeld H, ed. (2020). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Minostad 50 mg-Kapseln.
- "Principles and methods for the assessment of nephrotoxicity associated with exposure to chemicals" Archived 10 May 2011 at the Wayback Machine. Environmental health criteria: 119. World Health Organization (WHO). ISBN 92-4-157119-5. ISSN 0250-863X. 1991.
- Foye's Principles of Medicinal Chemistry; David A. Williams; William O. Foye, Thomas L. Lemke
- Goodman & Gilman's The Pharmacological Basis of Therapeutics, 12ed, Laurence L. Brunton, Bruce A. Chabner, Björn C. Knollmann
- Pfizer, Inc. v. International Rectifier Corp., 545 F. Supp. 486 (C.D. Cal. 1980) Archived 24 February 2015 at the Wayback Machine
- The Associated Press, 6 July 1983 "Pfizer to Get Rachelle Units" Archived 6 March 2016 at the Wayback Machine The New York Times.
- CDC Health Alert Network 12 June 2013 Nationwide Shortage of Doxycycline: Resources for Providers and Recommendations for Patient Care Archived 15 February 2015 at the Wayback Machine
- American Society of Health-System Pharmacists. 12 December 2014 Doxycycline Capsules and Tablets Archived 1 January 2015 at the Wayback Machine
- American Society of Health-System Pharmacists. 12 November 2014 Doxycycline Hyclate Injection Archived 1 January 2015 at the Wayback Machine
- Consumer Reports News: 4 February 2013 FDA reports shortage of doxycycline antibiotic. What are your options? Archived 1 January 2015 at the Wayback Machine
- Sudden increase in cost of common drug concerns many Archived 31 December 2014 at the Wayback Machine, WSMV-TV, 12 March 2013 (updated 26 March 2013).
- Rosenthal, Elisabeth, Officials Question the Rising Costs of Generic Drugs Archived 23 February 2017 at the Wayback Machine, The New York Times, 7 October 2014.
- Eric Palmer for FiercePharmaManufacturing. 13 March 2014 Hikma hits the jackpot with doxycycline shortage Archived 1 January 2015 at the Wayback Machine
- "Costco Drug Information". Archived from the original on 4 March 2016. Retrieved 31 July 2016.
- "Doxycycline Hyclate Prices and Doxycycline Hyclate Coupons". GoodRx. Archived from the original on 28 July 2016. Retrieved 31 July 2016.
- drugs.com Drugs.com international availability for doxycycline Archived 16 May 2015 at the Wayback Machine Page accessed 29 April 2015
- Leung E, Landa G (September 2013). "Update on current and future novel therapies for dry age-related macular degeneration". Expert Review of Clinical Pharmacology. 6 (5): 565–79. doi:10.1586/17512433.2013.829645. PMID 23971874. S2CID 26680094.
- Greenwald RA (December 2011). "The road forward: the scientific basis for tetracycline treatment of arthritic disorders". Pharmacological Research. 64 (6): 610–3. doi:10.1016/j.phrs.2011.06.010. PMID 21723947.
- Moullan N, Mouchiroud L, Wang X, Ryu D, Williams EG, Mottis A, et al. (March 2015). "Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research". Cell Reports. 10 (10): 1681–1691. doi:10.1016/j.celrep.2015.02.034. PMC 4565776. PMID 25772356.
- Chatzispyrou IA, Held NM, Mouchiroud L, Auwerx J, Houtkooper RH (November 2015). "Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research". Cancer Research. 75 (21): 4446–9. doi:10.1158/0008-5472.CAN-15-1626. PMC 4631686. PMID 26475870.
- Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H (June 1995). "Transcriptional activation by tetracyclines in mammalian cells". Science. 268 (5218): 1766–9. Bibcode:1995Sci...268.1766G. doi:10.1126/science.7792603. PMID 7792603.
- Dursun D, Kim MC, Solomon A, Pflugfelder SC (July 2001). "Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids". American Journal of Ophthalmology. 132 (1): 8–13. doi:10.1016/S0002-9394(01)00913-8. PMID 11438047.
- "Platform trial rules out treatments for COVID-19". NIHR Evidence. 31 May 2022. doi:10.3310/nihrevidence_50873.
- Butler CC, Yu LM, Dorward J, Gbinigie O, Hayward G, Saville BR, et al. (September 2021). "Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial". The Lancet. Respiratory Medicine. 9 (9): 1010–1020. doi:10.1016/S2213-2600(21)00310-6. PMC 8315758. PMID 34329624.
External links
- "Doxycycline". Drug Information Portal. U.S. National Library of Medicine.