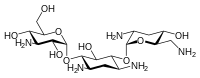
Tobramycin
Tobramycin is an aminoglycoside antibiotic derived from Streptomyces tenebrarius that is used to treat various types of bacterial infections, particularly Gram-negative infections. It is especially effective against species of Pseudomonas.[5]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nebcin, Tobrex, Tobi, others |
| Other names | 47663, SPRC-AB01 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682660 |
| License data |
|
| Pregnancy category | |
| Routes of administration | IV, IM, inhalation, eye drops/ointment |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Not bound[4] |
| Metabolism | Not metabolized |
| Elimination half-life | 2–3 hrs |
| Excretion | Exclusively via kidneys |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.642 |
| Chemical and physical data | |
| Formula | C18H37N5O9 |
| Molar mass | 467.515 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
It was patented in 1965, and approved for medical use in 1974.[6] It is on the World Health Organization's List of Essential Medicines.[7]
Medical uses
Like all aminoglycosides, tobramycin does not pass the gastro-intestinal tract, so for systemic use it can only be given intravenously or by injection into a muscle. Eye drops and ointments (tobramycin only, Tobrex, or combined with dexamethasone, sold as Tobradex) and nebulised formulations both have low systemic absorption. The formulation for injection is branded Nebcin. The nebulised formulation (brand name Tobi) is indicated in the treatment of exacerbations of chronic infection with Pseudomonas aeruginosa in people diagnosed with cystic fibrosis.[8][9]
Tobrex eye drops are a 0.3% tobramycin sterile ophthalmic solution produced by Alcon Pharmaceuticals. Benzalkonium chloride 0.01% is added as a preservative. It is available by prescription only in Bulgaria, Hungary, the United States, and Canada. In certain countries, it is available over the counter, It is also available in Egypt as Tobrin eye drops produced by EIPICo, Tobrex, Tobradex and Tobrin are indicated in the treatment of superficial infections of the eye, such as bacterial conjunctivitis.[10][11][12][13]
Tobramycin, in its injectable form, is also indicated for various severe or life-threatening infections caused by susceptible strains: sepsis, meningitis, lower respiratory tract infections, intra-abdominal infections, skin infections, bone infections, and skin structure infections, complicated and recurrent urinary tract infections.[14][3]
Spectrum of susceptibility
Tobramycin has a narrow spectrum of activity and is active against Gram-positive Staphylococcus aureus and various Gram-negative bacteria.[14] Clinically, tobramycin is frequently used to eliminate Pseudomonas aeruginosa in cystic fibrosis patients. The following represents the minimum inhibitory concentration (MIC) susceptibility data for a few strains of Pseudomonas aeruginosa:
- Pseudomonas aeruginosa - <0.25 µg/mL – 92 µg/mL [ref?]
- Pseudomonas aeruginosa (non-mucoid) – 0.5 µg/mL - >512 µg/mL [ref?]
- Pseudomonas aeruginosa (ATCC 27853) – 0.5 µg/mL – 2 µg/mL[15]
The MIC for Klebsiella pneumoniae, KP-1, is 2.3±0.2 µg/mL at 25 °C [unpublished].
Contraindications
Tobramycin is contraindicated in people with hypersensitivity against aminoglycoside antibiotics.[16] The Infusion is also contraindicated in people with myasthenia gravis.[4]
Side effects
Like other aminoglycosides, a major side effect for tobramycin is ototoxicity or a loss of equilibrioception, or both in genetically susceptible individuals.[17] Other side effects include nephrotoxicity, neuromuscular toxicity, and hypersensitivity reactions.[18] Nephrotoxicity can be particularly worrisome when multiple doses accumulate over the course of a treatment[19] or when the kidney concentrates urine by increasing tubular reabsorption during sleep. Adequate hydration may help prevent excess nephrotoxicity and subsequent loss of renal function.[20] For these reasons parenteral tobramycin needs to be carefully dosed by body weight, and its serum concentration monitored. Tobramycin is thus said to be a drug with a narrow therapeutic index.
Interactions
Muscle relaxants and diethylether can add to the neuromuscular blocking effects of tobramycin.[4]
Methoxyflurane, when used as an inhalational anesthetic, can aggravate the nephrotoxic effects of injected tobramycin. Likewise, combining injected tobramycin with other nephrotoxic or ototoxic drugs can lead to more adverse effects; examples include amphotericin B, ciclosporin, cisplatin, vancomycin, and the diuretic furosemide. Other diuretics can also increase the risk for side effects because they raise tobramycin concentrations in the body fluids.[4]
Combining tobramycin with betalactam antibiotics can be desirable because of their synergistic effects. However, when they are given through the same drip, as well as in people with reduced kidney function, they can react with each other to form antibiotically inactive amides.[4]
Pharmacology
Mechanism of action
Tobramycin works by binding to a site on the bacterial 30S and 50S ribosome, preventing formation of the 70S complex.[21] As a result, mRNA cannot be translated into protein, and cell death ensues.[22] Tobramycin also binds to RNA-aptamers,[23] artificially created molecules to bind to certain targets. However, there seems to be no indication that Tobramycin binds to natural RNAs or other nucleic acids.
The effect of tobramycin can be inhibited by metabolites of the Krebs (TCA) cycle, such as glyoxylate. These metabolites protect against tobramycin lethality by diverting carbon flux away from the TCA cycle, collapsing cellular respiration, and thereby inhibiting Tobramycin uptake and thus lethality.[24]
Pharmacokinetics
Tobramycin is not absorbed in the gut. When given as infusion, it is distributed in the extracellular fluid. It can accumulate in the kidney's tubular cells and in the lymph of the inner ear. Only low concentrations reach the central nervous system and breast milk. Tobramycin passes the placenta: in the fetus, 20% of the mother's concentrations have been measured.[4]
The substance is neither bound to plasma proteins, nor is it metabolized. It is excreted in unchanged form via the kidneys with a biological half-life of about 2 to 3 hours. Elimination from deep compartments such as the renal cortex follows after 8 to 12 hours. In newborns the half-life is 4.6 hours on average; in those with a low birth weight it is as long as 8.7 hours on average. People with reduced kidney function also have a longer half-life for tobramycin, while in those with severe burns it can be shorter.[4]
References
- "Tobramycin Use During Pregnancy". Drugs.com. 11 November 2019. Retrieved 28 April 2020.
- "Tobramycin ophthalmic Use During Pregnancy". Drugs.com. 24 December 2019. Retrieved 28 April 2020.
- "Tobramycin 40mg/ml Injection – Summary of Product Characteristics (SmPC)". (emc). 9 October 2018. Retrieved 28 April 2020.
- Haberfeld H, ed. (2021). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Tobramycin B. Braun 1 mg/ml Infusionslösung.
- "Tobramycin" (PDF). Toku-E. 12 January 2010. Retrieved 11 June 2012.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 507. ISBN 978-3-527-60749-5.
- World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- "Tobi- tobramycin solution". DailyMed. 5 October 2018. Retrieved 28 April 2020.
- "Tobi Podhaler". European Medicines Agency (EMA). 17 September 2018. Retrieved 28 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Tobrex- tobramycin ointment". DailyMed. 16 September 2019. Retrieved 28 April 2020.
- "Tobradex Eye Drops – Summary of Product Characteristics (SmPC)". (emc). 21 January 2020. Retrieved 28 April 2020.
- "Tobradex- tobramycin and dexamethasone ointment". DailyMed. 11 September 2019. Retrieved 28 April 2020.
- "Tobradex- tobramycin and dexamethasone suspension/ drops". DailyMed. 9 September 2019. Retrieved 28 April 2020.
- "Tobramycin- tobramycin sulfate injection, powder, for solution". DailyMed. 11 October 2019. Retrieved 28 April 2020.
- "Tobramycin Susceptibility and Minimum Inhibitory Concentration (MIC) Data" (PDF). Toku-E.
- Haberfeld H, ed. (2021). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Tobrex Augentropfen.
- Lerner AM, Reyes MP, Cone LA, Blair DC, Jansen W, Wright GE, Lorber RR (May 1983). "Randomised, controlled trial of the comparative efficacy, auditory toxicity, and nephrotoxicity of tobramycin and netilmicin". Lancet. 1 (8334): 1123–6. doi:10.1016/S0140-6736(83)92864-7. PMID 6133153. S2CID 8303933.
- Neu HC (August 1976). "Tobramycin: an overview". The Journal of Infectious Diseases. 134 (Suppl): S3–19. doi:10.1093/infdis/134.supplement_1.s3. JSTOR 30106949. PMID 787451.
- Pedersen SS, Jensen T, Osterhammel D, Osterhammel P (April 1987). "Cumulative and acute toxicity of repeated high-dose tobramycin treatment in cystic fibrosis". Antimicrobial Agents and Chemotherapy. 31 (4): 594–9. doi:10.1128/AAC.31.4.594. PMC 174783. PMID 3606063.
- Reyhanoglu G, Reddivari AK (January 2022). "Tobramycin". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 31869159.
- Yang G, Trylska J, Tor Y, McCammon JA (September 2006). "Binding of aminoglycosidic antibiotics to the oligonucleotide A-site model and 30S ribosomal subunit: Poisson-Boltzmann model, thermal denaturation, and fluorescence studies". Journal of Medicinal Chemistry. 49 (18): 5478–90. doi:10.1021/jm060288o. PMID 16942021.
- Haddad J, Kotra LP, Llano-Sotelo B, Kim C, Azucena EF, Liu M, et al. (April 2002). "Design of novel antibiotics that bind to the ribosomal acyltransfer site". Journal of the American Chemical Society. 124 (13): 3229–37. doi:10.1021/ja011695m. PMID 11916405.
- Kotra LP, Haddad J, Mobashery S (December 2000). "Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance". Antimicrobial Agents and Chemotherapy. 44 (12): 3249–56. doi:10.1128/aac.44.12.3249-3256.2000. PMC 90188. PMID 11083623.
- Meylan S, Porter CB, Yang JH, Belenky P, Gutierrez A, Lobritz MA, et al. (February 2017). "Carbon Sources Tune Antibiotic Susceptibility in Pseudomonas aeruginosa via Tricarboxylic Acid Cycle Control". Cell Chemical Biology. 24 (2): 195–206. doi:10.1016/j.chembiol.2016.12.015. PMC 5426816. PMID 28111098.
Further reading
- Davis BD (September 1987). "Mechanism of bactericidal action of aminoglycosides". Microbiological Reviews. 51 (3): 341–50. doi:10.1128/MMBR.51.3.341-350.1987. PMC 373115. PMID 3312985.
External links
- "Tobramycin". Drug Information Portal. U.S. National Library of Medicine.