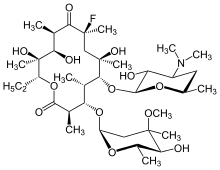
Flurithromycin
Flurithromycin is a second generation macrolide antibiotic. It is a fluorinated derivative of erythromycin A.[1] It is a broad spectrum antibiotic with similar bactericidal action to erythromycin. Unlike erythromycin, flurithromycin is more tolerant of acidic environments, meaning more survives the digestion process, resulting in higher serum levels, and more efficacious elimination of susceptible bacteria, including staphylococcus aureus and streptococcus pyogenes.[2]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.126.548 |
| Chemical and physical data | |
| Formula | C37H66FNO13 |
| Molar mass | 751.927 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
References
- Gialdroni Grassi G, Alesina R, Bersani C, Ferrara A, Fietta A, Peona V (June 1986). "In vitro activity of flurithromycin, a novel macrolide antibiotic". Chemioterapia: International Journal of the Mediterranean Society of Chemotherapy. 5 (3): 177–84. PMID 3487389.
- Kaneko, T.; Dougherty, T. J.; Magee, T. V. (2007-01-01), Taylor, John B.; Triggle, David J. (eds.), "7.18 - Macrolide Antibiotics", Comprehensive Medicinal Chemistry II, Oxford: Elsevier, pp. 519–566, doi:10.1016/b0-08-045044-x/00219-4, ISBN 978-0-08-045044-5, retrieved 2022-08-03
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.