Pristinamycin IIA
 | |
| Clinical data | |
|---|---|
| Other names | Mikamycin A; Virginiamycin M1 |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H35N3O7 |
| Molar mass | 525.602 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Introduction
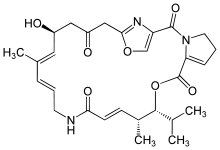
Pristinamycin IIA is a macrolide antibiotic. It is a member of the streptogramin A group of antibiotics and one component of pristinamycin (the other being pristinamycin IA).[1] Pristinamycin IIA was first isolated from the Streptomyces virginiae, but has been isolated from other microorganisms and thus has been given a variety of other names such as Virginiamycin M1, Mikamycin A, and Streptogramin A.[2] Pristinamycin IIA structure was determined by chemical and instrumental techniques, including X-ray crystallography.[2][3] Pristinamycin IIA is of interest from a biosynthetic viewpoint because it contains the unusual dehydroproline and oxazole ring systems.[2] The only experimental evidence bearing on the formation of the oxazole ring is found in work on the biosynthesis of the alkaloid annuloline.[2][4]
Biosynthesis
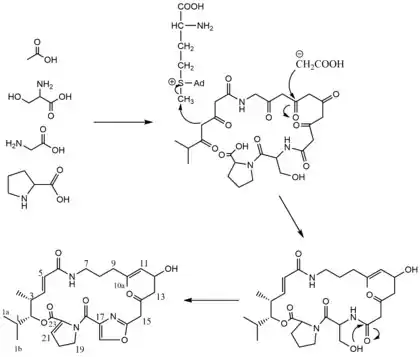
Pristinamycin IIA biosynthesis is presumed to proceed through the acetate pathway and was determined through the feeding of 3H and 13C precursors to Streptomyces virginiae strain PDT-30.[2] When fed [2-13C]-acetate the 13C NMR Spectra showed signals corresponding to carbons 5, 9, 10a, 11, 13, and 15 seen in the biosynthesis scheme.[2] In addition, methionine was found to donate its methyl group specifically to carbon-3 (seen in the biosynthesis scheme) by studies with L-[methyl-13C] methionine.[2] With this data and the known incorporation of proline, methionine, serine, and glycine into the antibiotic along with the assumption that carbon atoms 1, la, lb, and 2 are derived from valine or isobutyric acid, allows for a tentative pathway for the biosynthesis of Pristinamycin IIA to be deduced.[2]
See also
References
- Laforest H, Fourgeaud M, Richet H, Lagrange PH (July 1988). "Comparative in vitro activities of pristinamycin, its components, and other antimicrobial agents against anaerobic bacteria". Antimicrobial Agents and Chemotherapy. 32 (7): 1094–6. doi:10.1128/AAC.32.7.1094. PMC 172352. PMID 3142342.
- Kingston, David; Kolpak, Michael (August 1980). "Biosynthesis of antibiotics of the virginiamycin family. 1. Biosynthesis of virginiamycin M1: determination of the labeling pattern by the use of stable isotope techniques". Journal of the American Chemical Society. 102 (18): 5964–5966. doi:10.1021/ja00538a070. Retrieved 29 May 2022.
- Delpierre, G. R.; Eastwood, F.W.; Gream, G.E.; Kingston, D.G.; Sarin, P.S.; Todd, Lord; Williams, D.H. (1966). "Antibiotics of the ostreogrycin complex. Part II. Structure of ostreogrycin A". Journal of the Chemical Society C: Organic. 1 (0): 1653–1669. doi:10.1039/j39660001653. Retrieved 29 May 2022.
- O’Donovan, D.G.; Horan, H.J (1971). "The biosynthesis of annuloline, a unique oxazole alkaloid". Journal of the Chemical Society C: Organic. 1 (0): 331–334. doi:10.1039/J39710000331. Retrieved 29 May 2022.