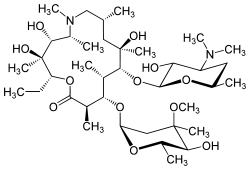
Azithromycin
Azithromycin is an antibiotic medication used for the treatment of a number of bacterial infections.[4] This includes middle ear infections, strep throat, pneumonia, traveler's diarrhea, and certain other intestinal infections.[4] Along with other medications, it may also be used for malaria.[4] It can be taken by mouth or intravenously with doses once per day.[4]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zithromax, Azithrocin, Sumamed, others[1] |
| Other names | 9-deoxy-9α-aza-9α-methyl-9α-homoerythromycin A |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697037 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (capsule, tablet or suspension), intravenous, eye drop |
| Drug class | Macrolide antibiotic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 38% for 250 mg capsules |
| Metabolism | Liver |
| Elimination half-life | 11–14 h (single dose) 68 h (multiple dosing) |
| Excretion | Bile duct, kidney (4.5%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.126.551 |
| Chemical and physical data | |
| Formula | C38H72N2O12 |
| Molar mass | 748.996 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| GLAH&page2=Azithromycin (verify) | |
Common side effects include nausea, vomiting, diarrhea and upset stomach.[4] An allergic reaction, such as anaphylaxis, QT prolongation, or a type of diarrhea caused by Clostridium difficile is possible.[4] No harm has been found with its use during pregnancy.[4] Its safety during breastfeeding is not confirmed, but it is likely safe.[5] Azithromycin is an azalide, a type of macrolide antibiotic.[4] It works by decreasing the production of protein, thereby stopping bacterial growth.[4][6]
Azithromycin was discovered in 1980 by the Croatian pharmaceutical company Pliva and approved for medical use under the brand name Sumamed in 1988.[7][8] It is on the World Health Organization's List of Essential Medicines.[9] The World Health Organization classifies it as critically important for human medicine.[10] It is available as a generic medication[11] and is sold under many trade names worldwide.[1] In 2020, it was the 68th most commonly prescribed medication in the United States, with more than 10 million prescriptions.[12][13]
Medical uses
Azithromycin is used to treat diverse infections, including:
- Prevention and treatment of acute bacterial exacerbations of chronic obstructive pulmonary disease due to H. influenzae, M. catarrhalis, or S. pneumoniae. The benefits of long-term prophylaxis must be weighed on a patient-by-patient basis against the risk of cardiovascular and other adverse effects.[14]
- Community-acquired pneumonia due to C. pneumoniae, H. influenzae, M. pneumoniae, or S. pneumoniae[15]
- Uncomplicated skin infections due to S. aureus, S. pyogenes, or S. agalactiae
- Urethritis and cervicitis due to C. trachomatis or N. gonorrhoeae. In combination with ceftriaxone, azithromycin is part of the United States Centers for Disease Control-recommended regimen for the treatment of gonorrhea. Azithromycin is active as monotherapy in most cases, but the combination with ceftriaxone is recommended based on the relatively low barrier to resistance development in gonococci and due to frequent co-infection with C. trachomatis and N. gonorrhoeae.[16]
- Trachoma due to C. trachomatis[17]
- Genital ulcer disease (chancroid) in men due to H. ducrey
- Acute bacterial sinusitis due to H. influenzae, M. catarrhalis, or S. pneumoniae. Other agents, such as amoxicillin/clavulanate are generally preferred, however.[18][19]
- Acute otitis media caused by H. influenzae, M. catarrhalis or S. pneumoniae. Azithromycin is not, however, a first-line agent for this condition. Amoxicillin or another beta lactam antibiotic is generally preferred.[20]
- Pharyngitis or tonsillitis caused by S. pyogenes as an alternative to first-line therapy in individuals who cannot use first-line therapy[21]

Bacterial susceptibility
Azithromycin has relatively broad but shallow antibacterial activity. It inhibits some Gram-positive bacteria, some Gram-negative bacteria, and many atypical bacteria.
A strain of gonorrhea reported to be highly resistant to azithromycin was found in the population in 2015. Neisseria gonorrhoeae is normally susceptible to azithromycin,[22] but the drug is not widely used as monotherapy due to a low barrier to resistance development.[16] Extensive use of azithromycin has resulted in growing Streptococcus pneumoniae resistance.[23]
Aerobic and facultative Gram-positive microorganisms
- Staphylococcus aureus (Methicillin-sensitive only)
- Streptococcus agalactiae
- Streptococcus pneumoniae
- Streptococcus pyogenes
Aerobic and facultative anaerobic Gram-negative microorganisms
- Haemophilus ducreyi
- Haemophilus influenzae
- Moraxella catarrhalis
- Neisseria gonorrhoeae
- Bordetella pertussis
- Legionella pneumophila
Anaerobic microorganisms
- Peptostreptococcus species
- Prevotella bivia
Other microorganisms
Pregnancy and breastfeeding
No harm has been found with use during pregnancy.[4] However, there are no adequate well-controlled studies in pregnant women.[24]
The safety of the medication during breastfeeding is unclear. It was reported that because only low levels are found in breast milk and the medication has also been used in young children, it is unlikely that breastfed infants would have adverse effects.[5] Nevertheless, it is recommended that the drug be used with caution during breastfeeding.[4]
Airway diseases
Azithromycin appears to be effective in the treatment of chronic obstructive pulmonary disease through its suppression of inflammatory processes.[25] And potentially useful in asthma and sinusitis via this mechanism.[26] Azithromycin is believed to produce its effects through suppressing certain immune responses that may contribute to inflammation of the airways.[27][28]
Adverse effects
Most common adverse effects are diarrhea (5%), nausea (3%), abdominal pain (3%), and vomiting. Fewer than 1% of people stop taking the drug due to side effects. Nervousness, skin reactions, and anaphylaxis have been reported.[29] Clostridium difficile infection has been reported with use of azithromycin.[4] Azithromycin does not affect the efficacy of birth control unlike some other antibiotics such as rifampin. Hearing loss has been reported.[30]
Occasionally, people have developed cholestatic hepatitis or delirium. Accidental intravenous overdose in an infant caused severe heart block, resulting in residual encephalopathy.[31][32]
In 2013 the FDA issued a warning that azithromycin "can cause abnormal changes in the electrical activity of the heart that may lead to a potentially fatal irregular heart rhythm." The FDA noted in the warning a 2012 study that found the drug may increase the risk of death, especially in those with heart problems, compared with those on other antibiotics such as amoxicillin or no antibiotic. The warning indicated people with preexisting conditions are at particular risk, such as those with QT interval prolongation, low blood levels of potassium or magnesium, a slower than normal heart rate, or those who use certain drugs to treat abnormal heart rhythms.[33][34][35]
It has been reported that azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection.[36]
Pharmacology
Mechanism of action
Azithromycin prevents bacteria from growing by interfering with their protein synthesis. It binds to the 50S subunit of the bacterial ribosome, thus inhibiting translation of mRNA. Nucleic acid synthesis is not affected.[24]
Pharmacokinetics
Azithromycin is an acid-stable antibiotic, so it can be taken orally with no need of protection from gastric acids. It is readily absorbed, but absorption is greater on an empty stomach. Time to peak concentration (Tmax) in adults is 2.1 to 3.2 hours for oral dosage forms. Due to its high concentration in phagocytes, azithromycin is actively transported to the site of infection. During active phagocytosis, large concentrations are released. The concentration of azithromycin in the tissues can be over 50 times higher than in plasma due to ion trapping and its high lipid solubility. Azithromycin's half-life allows a large single dose to be administered and yet maintain bacteriostatic levels in the infected tissue for several days.[37]
Following a single dose of 500 mg, the apparent terminal elimination half-life of azithromycin is 68 hours.[37] Biliary excretion of azithromycin, predominantly unchanged, is a major route of elimination. Over the course of a week, about 6% of the administered dose appears as an unchanged drug in urine.
History
A team of researchers at the pharmaceutical company Pliva in Zagreb, Croatia,—Gabrijela Kobrehel, Gorjana Radobolja-Lazarevski, and Zrinka Tamburašev, led by Slobodan Đokić—discovered azithromycin in 1980.[38] The company Pliva patented it in 1981.[8] In 1986, Pliva and Pfizer signed a licensing agreement, which gave Pfizer exclusive rights for the sale of azithromycin in Western Europe and the United States. Pliva put its azithromycin on the market in Central and Eastern Europe under the brand name Sumamed in 1988. Pfizer launched azithromycin under Pliva's license in other markets under the brand name Zithromax in 1991.[39] Patent protection ended in 2005.[40]
Society and culture
_tablets.jpg.webp)
Available forms
Azithromycin is available as a generic medication. Azithromycin is commonly administered in film-coated tablet, capsule, oral suspension, intravenous injection, granules for suspension in sachet, and ophthalmic solution.[1]
Usage
In 2010, azithromycin was the most prescribed antibiotic for outpatients in the US,[41] whereas in Sweden, where outpatient antibiotic use is a third as prevalent, macrolides are only on 3% of prescriptions.[42] In 2017, azithromycin was the second most prescribed antibiotic for outpatients in the United States.[43]
Brand name listings |
|---|
|
It is sold under many trade names worldwide including 3-Micina, A Sai Qi, Abacten, Abbott, Acex, Acithroc, Actazith, Agitro, Ai Mi Qi, Amixef, Amizin, Amovin, An Mei Qin, Ao Li Ping, Apotex, Lebanon, Aratro, Aruzilina, Arzomicin, Arzomidol, Asizith, Asomin, Astidal, Astro, Athofix, Athxin, Atizor, Atromizin, Avalon, AZ, AZA, Azacid, Azadose, Azalid, Azalide, AzaSite, Azath, Azatril, Azatril, Azax, Azee, Azeecor, Azeeta, Azelide, Azeltin, Azenil, Azeptin, Azerkym, Azi, Aziact, Azibact, Azibactron, Azibay, Azibect, Azibest, Azibiot, Azibiotic, Azicare, Azicin, Azicine, Aziclass, Azicom, Azicure, Azid, Azidose, Azidraw, Azifam, Azifarm, Azifast, Azifine, Azigen, Azigram, Azigreat, Azikare, Azilide, Azilife, Azilip, Azilup, Azimac, Azimax, Azimed, Azimepha, Azimex, Azimit, Azimix, Azimon, Azimore, Azimycin, Azimycine, Azin, Azindamon, Azinew, Azinex, Azinif, Azinil, Azintra, Aziom, Azipar, Aziped, Aziphar, Azipin, Azipro, Aziprome, Aziquilab, Azirace, Aziram, Aziresp, Aziride, Azirol, Azirom, Azirox, Azirute, Azirutec, Aziset, Azisis, Azison, Azissel, Aziswift, Azit, Azita, Azitam, Azitex, Azith, Azithral, Azithrin, Azithro, Azithrobeta, Azithrocin, Azithrocine, Azithromax, Azithromed, Azithromicina, Azithromycin, Azithromycine, Azithromycinum, Azithrovid, Azitic, Azitive, Azitome, Azitrac, Azitral, Azitrax, Azitredil, Azitrex, Azitrim, Azitrin, Azitrix, Azitro, Azitrobac, Azitrocin, Azitroerre, Azitrogal, Azitrolabsa, Azitrolid, Azitrolit, Azitrom, Azitromac, Azitromax, Azitromek, Azitromicin, Azitromicina, Azitromycin, Azitromycine, Azitrona, Azitropharma, Azitroteg, Azitrox, Azitsa, Azitus, Azivar, Azivirus, Aziwill, Aziwok, Azix, Azizox, Azmycin, Azo, Azobat, Azocin, Azoget, Azoheim, Azoksin, Azom, Azomac, Azomax, Azomex, Azomycin, Azomyne, Azores, Azorox, Azostar, Azot, Azoxin, Azras, Azro, Azrocin, Azrolid, Azromax, Azrosin, Aztin, Aztrin, Aztro, Aztrogecin, Azvig, Azycin, Azycyna, Azydrop, Azypin, Azytact, Azytan, Azyter, Azyter, Azyth, Azywell, Azza, Ba Qi, Bactizith, Bactrazol, Bai Ke De Rui, Batif, Bazyt, Bezanin, Bin Qi, Binozyt, BinQi, Biocine, Biozit, Bo Kang, Canbiox, Cetaxim, Charyn, Chen Yu, Cinalid, Cinetrin, Clamelle, Clearsing, Corzi, Cozith, Cronopen, Curazith, Delzosin, Dentazit, Disithrom, Doromax, Doyle, Elzithro, Eniz, Epica, Ethrimax, Ezith, Fabodrox, Fabramicina, Feng Da Qi, Figothrom, Floctil, Flumax, Fu Qi-Hua Yuan, Fu Rui Xin, Fuqixing, Fuxin-Hai Xin Pharm, Geozif, Geozit, Gitro, Goldamycin, Gramac, Gramokil, Hemomicin, Hemomycin, I-Thro, Ilozin, Imexa, Inedol, Infectomycin, Iramicina, Itha, Jin Nuo, Jin Pai Qi, Jinbo, Jun Jie, Jun Wei Qing, Kai Qi, Kang Li Jian, Kang Qi, Katrozax, Ke Lin Da, Ke Yan Li, Koptin, Kuai Yu, L-Thro, Laz, Legar, Lg-Thral, Li Ke Si, Li Li Xing, Li Qi, Lin Bi, Lipuqi, Lipuxin, Lizhu Qile, Loromycin, Lu Jia Kang, Luo Bei Er, Luo Qi, Maazi, Macroazi, Macromax, Macrozit, Maczith, Makromicin, Maxmor, Mazit, Mazitrom, Medimacrol, Meithromax, Mezatrin, Ming Qi Xin, Misultina, Na Qi, Nadymax, Naxocina, Neblic, Nemezid, Neofarmiz, Nifostin, Nobaxin, Nokar, Novatrex, Novozithron, Novozitron, Nurox, Odaz, Odazyth, Onzet, Oranex, Oranex, Ordipha, Orobiotic, Pai Fen, Pai Fu, Paiqi, Pediazith, Portex, Pu He, Pu Le Qi, Pu Yang, Qi Gu Mei, Qi Mai Xing, Qi Nuo, Qi Tai, Qi Xian, Qili, Qiyue, Rarpezit, Razimax, Razithro, Rezan, Ribotrex, Ribozith, Ricilina, Rizcin, Romycin, Rothin (Rakaposhi), Rozalid, Rozith, Ru Shuang Qi, Rui Qi, Rui Qi Lin, Rulide, Sai Jin Sha, Sai Le Xin, Sai Qi, Selimax, Sheng Nuo Ling, Shu Luo Kang, Simpli-3, Sisocin, Sitrox, Sohomac, Stromac, Su Shuang, Sumamed, Sumamox, Tailite, Talcilina, Tanezox, Te Li Xin, Tetris, Texis, Thoraxx, Throin, Thromaxin, Tong Tai Qi Li, Topt, Toraseptol, Tremac, Trex, Tri Azit, Triamid, Tridosil, Trimelin, Tritab, Tromiatlas, Tromix, Trozamil, Trozin, Trozocina, Trulimax, Tuoqi, Udox, Ultreon, Ultreon, Vectocilina, Vinzam, Visag, Vizicin, Wei Li Qinga, Wei Lu De, Wei Zong, Weihong, Xerexomair, Xi Le Xin, Xi Mei, Xin Da Kang, Xin Pu Rui, Xithrone, Ya Rui, Yan Sha, Yanic, Yi Nuo Da, Yi Song, Yi Xina, Yin Pei Kang, Yong Qi, You Ni Ke, Yu Qi, Z-3, Z-PAK, Zady, Zaiqi, Zaret, Zarom, Zathrin, Zedbac, Zeemide, Zenith, Zentavion, Zetamac, Zetamax, Zeto, Zetron, Zevlen, Zibramax, Zicho, Zigilex, Zikti, Zimacrol, Zimax, Zimicina, Zindel, Zinfect, Zirom, Zisrocin, Zistic, Zit-Od, Zitab, Zitax, Zithrax, Zithrin, Zithro-Due, Zithrobest, Zithrodose, Zithrogen, Zithrokan, Zithrolide, Zithromax, Zithrome, Zithromed, Zithroplus, Zithrotel, Zithrox, Zithroxyn, Zithtec, Zitinn, Zitmac, Zitraval, Zitrax, Zitrex, Zitric, Zitrim, Zitrobid, Zitrobiotic, Zithrolect, Zitrocin, Zitrocin, Zitrogram, Zitrolab, Zitromax, Zitroneo, Zitrotek, Ziyoazi, Zmax, Zocin, Zomax, Zotax, Zycin, and Zythrocin.[1] It is sold as a combination drug with cefixime as Anex-AZ, Azifine-C, Aziter-C, Brutacef-AZ, Cezee, Fixicom-AZ, Emtax-AZ, Olcefone-AZ, Starfix-AZ, Zeph-AZ, Zicin-CX, and Zifi-AZ.[1] It is also sold as a combination drug with nimesulide as Zitroflam; in a combination with tinidazole and fluconazole as Trivafluc, and in a combination with ambroxol as Zathrin-AX, Laz-AX and Azro-AM.[1] |
Research
COVID-19
Despite early evidence showing azithromycin slowed down coronavirus multiplication in laboratory settings, further research indicates it to be ineffective as a treatment for COVID-19 in humans.[44] After a large-scale trial showed no benefit of using azithromycin in treating COVID-19, the UK's National Institute for Health and Care Excellence (NICE) updated its guidance and no longer recommends the medication for COVID-19.[45]
References
- "Azithromycin International Brands". Drugs.com. Archived from the original on 28 February 2017. Retrieved 27 February 2017.
- "Azithromycin Use During Pregnancy". Drugs.com. 2 May 2019. Retrieved 24 December 2019.
- https://www.ema.europa.eu/documents/psusa/azithromycin-list-nationally-authorised-medicinal-products-psusa/00010491/202004_en.pdf
- "Azithromycin". The American Society of Health-System Pharmacists. Archived from the original on 5 September 2015. Retrieved 1 August 2015.
- "Azithromycin use while Breastfeeding". Archived from the original on 5 September 2015. Retrieved 4 September 2015.
- "Azithromycin Stops The Growth of Bacteria" (in German). Retrieved 24 December 2017.
- Greenwood, David (2008). Antimicrobial drugs : chronicle of a twentieth century medical triumph (1. publ. ed.). Oxford: Oxford University Press. p. 239. ISBN 9780199534845. Archived from the original on 5 March 2016.
- Alapi EM, Fischer J (2006). "Table of Selected Analogue Classes". In Fischer J, Ganellin CR (eds.). Analogue-based Drug Discovery. Weinheim: Wiley-Vch Verlag GmbH & Co. KGaA. p. 498. ISBN 978-3-527-31257-3.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- World Health Organization (2018). Critically important antimicrobials for human medicine (6th revision ed.). Geneva: World Health Organization. hdl:10665/312266. ISBN 9789241515528. License: CC BY-NC-SA 3.0 IGO. Archived from the original on 22 October 2019.
- Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. ISBN 9781284057560.
- "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- "Azithromycin - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- Taylor SP, Sellers E, Taylor BT (December 2015). "Azithromycin for the Prevention of COPD Exacerbations: The Good, Bad, and Ugly". The American Journal of Medicine. 128 (12): 1362.e1–6. doi:10.1016/j.amjmed.2015.07.032. PMID 26291905.
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. (March 2007). "Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults". Clinical Infectious Diseases. 44 Suppl 2: S27-72. doi:10.1086/511159. PMC 7107997. PMID 17278083.
- "Gonococcal Infections - 2015 STD Treatment Guidelines". Archived from the original on 1 March 2016.
- Burton M, Habtamu E, Ho D, Gower EW (November 2015). "Interventions for trachoma trichiasis". The Cochrane Database of Systematic Reviews. 11 (11): CD004008. doi:10.1002/14651858.CD004008.pub3. PMC 4661324. PMID 26568232.
- Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, et al. (April 2015). "Clinical practice guideline (update): adult sinusitis". Otolaryngology–Head and Neck Surgery. 152 (2 Suppl): S1–S39. doi:10.1177/0194599815572097. PMID 25832968.
- Hauk L (April 2014). "AAP releases guideline on diagnosis and management of acute bacterial sinusitis in children one to 18 years of age". American Family Physician. 89 (8): 676–81. PMID 24784128.
- Neff MJ (June 2004). "AAP, AAFP release guideline on diagnosis and management of acute otitis media". American Family Physician. 69 (11): 2713–5. PMID 15202704.
- Randel A (September 2013). "IDSA Updates Guideline for Managing Group A Streptococcal Pharyngitis". American Family Physician. 88 (5): 338–40. PMID 24010402.
- The Guardian newspaper: 'Super-gonorrhoea' outbreak in Leeds, 18 September 2015 Archived 18 September 2015 at the Wayback Machine
- Lippincott Illustrated Reviews : Pharmacology Sixth Edition. p. 506.
- "US azithromycin label" (PDF). FDA. February 2016. Archived (PDF) from the original on 23 November 2016.
- Simoens S, Laekeman G, Decramer M (May 2013). "Preventing COPD exacerbations with macrolides: a review and budget impact analysis". Respiratory Medicine. 107 (5): 637–48. doi:10.1016/j.rmed.2012.12.019. PMID 23352223.
- Gotfried MH (February 2004). "Macrolides for the treatment of chronic sinusitis, asthma, and COPD". Chest. 125 (2 Suppl): 52S–60S, quiz 60S-61S. doi:10.1378/chest.125.2_suppl.52S. PMID 14872001.
- Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K (May 2012). "Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases". European Journal of Clinical Pharmacology. 68 (5): 479–503. doi:10.1007/s00228-011-1161-x. PMID 22105373. S2CID 1904304.
- Steel HC, Theron AJ, Cockeran R, Anderson R, Feldman C (2012). "Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics". Mediators of Inflammation. 2012: 584262. doi:10.1155/2012/584262. PMC 3388425. PMID 22778497.
- Mori F, Pecorari L, Pantano S, Rossi ME, Pucci N, De Martino M, Novembre E (2014). "Azithromycin anaphylaxis in children". International Journal of Immunopathology and Pharmacology. 27 (1): 121–6. doi:10.1177/039463201402700116. PMID 24674687. S2CID 45729751.
- Dart RC (2004). Medical Toxology. Lippincott Williams & Wilkins. p. 23.
- Tilelli JA, Smith KM, Pettignano R (January 2006). "Life-threatening bradyarrhythmia after massive azithromycin overdose". Pharmacotherapy. 26 (1): 147–50. doi:10.1592/phco.2006.26.1.147. PMID 16506357. S2CID 43222966.
- Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 132–133.
- Grady D (16 May 2012). "Popular Antibiotic May Raise Risk of Sudden Death". The New York Times. Archived from the original on 17 May 2012. Retrieved 18 May 2012.
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM (May 2012). "Azithromycin and the risk of cardiovascular death". The New England Journal of Medicine. 366 (20): 1881–90. doi:10.1056/NEJMoa1003833. PMC 3374857. PMID 22591294.
- "FDA Drug Safety Communication: Azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms". FDA. 12 March 2013. Archived from the original on 27 October 2016.
- Maurizio R (September 2011). "Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection". J Clin Invest. 121 (9): 3554–63. doi:10.1172/JCI46095. PMC 3163956. PMID 21804191.
- "Zithromax - FDA prescribing information, side effects and uses". Archived from the original on 14 October 2014. Retrieved 10 October 2014.
- Banić Tomišić Z (December 2011). "The Story of Azithromycin". Kemija U Industriji: Časopis Kemičara I Kemijskih Inženjera Hrvatske. 60 (12): 603–17.
- Banić Tomišić Z (2011). "The Story of Azithromycin". Kemija U Industriji. 60 (12): 603–617. ISSN 0022-9830. Archived from the original on 8 September 2017. Retrieved 15 April 2013.
- "Azithromycin: A world best-selling Antibiotic". www.wipo.int. World Intellectual Property Organization. Retrieved 18 June 2019.
- Hicks LA, Taylor TH, Hunkler RJ (April 2013). "U.S. outpatient antibiotic prescribing, 2010". The New England Journal of Medicine. 368 (15): 1461–2. doi:10.1056/NEJMc1212055. PMID 23574140.
- Hicks LA, Taylor TH, Hunkler RJ (September 2013). "More on U.S. outpatient antibiotic prescribing, 2010". The New England Journal of Medicine. 369 (12): 1175–6. doi:10.1056/NEJMc1306863. PMID 24047077.
- "Outpatient Antibiotic Prescriptions - United States, 2017 - Community - Antibiotic Use". Centers for Disease Control and Prevention (CDC). 26 March 2020. Retrieved 30 March 2020.
- Popp M, Stegemann M, Riemer M, Metzendorf M, Romero CS, Mikolajewska A, Kranke P, Meybohm P, Skoetz N, Weibel S (22 October 2021). "Intervention Antibiotics for the treatment of COVID‐19". Cochrane Database of Systematic Reviews. 10: CD015025. doi:10.1002/14651858.CD015025. PMC 8536098. PMID 34679203.
- "Platform trial rules out treatments for COVID-19". NIHR Evidence (Plain English summary). 31 May 2022. doi:10.3310/nihrevidence_50873.
External links
- "Azithromycin". Drug Information Portal. U.S. National Library of Medicine.