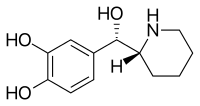
Rimiterol
Rimiterol (INN/USAN) is a third-generation[1] short-acting[2][3] β2 agonist.
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.627 |
| Chemical and physical data | |
| Formula | C12H17NO3 |
| Molar mass | 223.272 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
See also
References
- Palma-Carlos AG, Palma-Carlos GS (April 1986). "Beta-2-agonists of third generation". Allergie et Immunologie. 18 (4): 31–2, 35. PMID 2899434.
- Lai CK, Twentyman OP, Holgate ST (October 1989). "The effect of an increase in inhaled allergen dose after rimiterol hydrobromide on the occurrence and magnitude of the late asthmatic response and the associated change in nonspecific bronchial responsiveness". The American Review of Respiratory Disease. 140 (4): 917–23. doi:10.1164/ajrccm/140.4.917. PMID 2572192.
- Ydreborg SO, Svedmyr N, Thiringer G (April 1977). "Comparison of rimiterol and terbutaline, given by aerosol, in a long-term study". Scandinavian Journal of Respiratory Diseases. 58 (2): 117–24. PMID 16339.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.