Esmirtazapine
Esmirtazapine (ORG-50,081) is a drug which was under development by Organon for the treatment of insomnia and vasomotor symptoms (e.g., hot flashes) associated with menopause.[3][4][5][6] Esmirtazapine is the (S)-(+)-enantiomer of mirtazapine and possesses similar overall pharmacology, including inverse agonist actions at H1 and 5-HT2 receptors and antagonist actions at α2-adrenergic receptors.[3][7]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver (CYP2D6)[1] |
| Elimination half-life | 10 hours[2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ECHA InfoCard | 100.056.994 |
| Chemical and physical data | |
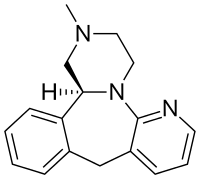
| Formula | C17H19N3 |
| Molar mass | 265.360 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 114 to 116 °C (237 to 241 °F) |
| Solubility in water | Soluble in methanol and chloroform mg/mL (20 °C) |
SMILES
| |
InChI
| |
| | |
Notably, esmirtazapine has a shorter half life of around 10 hours, compared to R-mirtazapine and racemic mixture, which has a half-life of 18-40 hours.[2] Merck has run several studies on low dose (3 - 4.5 mg) esmirtazapine for the treatment of insomnia. It is attractive for treating insomnia since it is a potent H1-inhibitor and a 5-HT2A antagonist.[8][2] Unlike low-dose mirtazapine, the half life (10 hours) is short enough that next-day sedation may be manageable, unfortunately however, for people with CYP2D6 polymorphisms, which constitute a sizable fraction of the population, the half-life is expected to be quite a bit longer. Merck researchers claimed that the incidence of next-day sedation was not a problem in one of their studies, but this claim has been challenged (15% of patients complained of daytime sleepiness vs 3.5% in the placebo group).[9]
In March 2010, Merck terminated its internal clinical development program for esmirtazapine for hot flashes and insomnia, "for strategic reasons".[10]
See also
References
- "A population analysis on the effects of the CYP2D6 deficiency on pharmacokinetics and exposure of esmirtazapine in healthy volunteers" (PDF).
- Ruwe, Frank; IJzerman-Boon, Pieta; Roth, Thomas; Zammit, Gary; Ivgy-May, Neely (October 2016). "A Phase 2 Randomized Dose-Finding Study With Esmirtazapine in Patients With Primary Insomnia". Journal of Clinical Psychopharmacology. 36 (5): 457–464. doi:10.1097/JCP.0000000000000546. PMID 27482970. S2CID 25639396.
- "Future Treatments for Depression, Anxiety, Sleep Disorders, Psychosis, and ADHD -- Neurotransmitter.net".
- "A Long-Term Safety Study of Org 50081 in Elderly Outpatients With Chronic Primary Insomnia (176005)(P05697) - Full Text View - ClinicalTrials.gov". 7 January 2021.
{{cite journal}}: Cite journal requires|journal=(help) - Teegarden BR, Al Shamma H, Xiong Y (2008). "5-HT(2A) inverse-agonists for the treatment of insomnia". Current Topics in Medicinal Chemistry. 8 (11): 969–76. doi:10.2174/156802608784936700. PMID 18673166.
- Lewis V (November 2009). "Undertreatment of menopausal symptoms and novel options for comprehensive management". Current Medical Research and Opinion. 25 (11): 2689–98. doi:10.1185/03007990903240519. PMID 19775194. S2CID 206964530.
- Depression and bipolar disorder: Stahl's essential psychopharmacology. Cambridge, UK: Cambridge University Press. 2008. ISBN 978-0-521-88663-5.
- Ivgy-May, Neely; Ruwe, Frank; Krystal, Andrew; Roth, Thomas (1 July 2015). "Esmirtazapine in non-elderly adult patients with primary insomnia: efficacy and safety from a randomized, 6-week sleep laboratory trial". Sleep Medicine. 16 (7): 838–844. doi:10.1016/j.sleep.2015.04.001. PMID 26047892.
- Ivgy-May, Neely; Hajak, Goeran; van Osta, Gonnie; Braat, Sabine; Chang, Qing; Roth, Thomas (15 September 2020). "Efficacy and safety of esmirtazapine in adult outpatients with chronic primary insomnia: a randomized, double-blind placebo-controlled study and open-label extension". Journal of Clinical Sleep Medicine. 16 (9): 1455–1467. doi:10.5664/jcsm.8526. PMC 7970588. PMID 32351205.
- "Archived copy" (PDF). Archived from the original (PDF) on 2011-08-05. Retrieved 2011-05-03.
{{cite web}}: CS1 maint: archived copy as title (link)
External links
 Media related to Esmirtazapine at Wikimedia Commons
Media related to Esmirtazapine at Wikimedia Commons
| GABAA |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GABAB | |||||||||||||||||||||||||
| H1 |
| ||||||||||||||||||||||||
| α2-Adrenergic |
| ||||||||||||||||||||||||
| 5-HT2A |
| ||||||||||||||||||||||||
| Melatonin | |||||||||||||||||||||||||
| Orexin | |||||||||||||||||||||||||
| α2δ VDCC | |||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Classes |
|
|---|---|
| Antidepressants (TCAs and TeCAs) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Others |
|