Promazine
Promazine (brand name Sparine among others),[1] is used as a short-term add-on treatment for psychomotor agitation.[2][3] Its approved uses in people is limited, but is used as a tranquilizer in veterinary medicine.[2] It has weak antipsychotic effects but is generally not used to treat psychoses.[2]
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a600010 |
| Drug class | Typical antipsychotic |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 94% |
| Elimination half-life | 20-40 hr |
| Identifiers | |
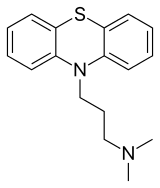
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.347 |
| Chemical and physical data | |
| Formula | C17H20N2S |
| Molar mass | 284.42 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
It acts similar to chlorpromazine and causes sedation.[2] It has predominantly anticholinergic side effects, though extrapyramidal side effects are not uncommon. It belongs to the typical antipsychotic and phenothiazine class of drugs.[4]
Promazine was approved for medical use in the United States in the 1950s, although it is no longer commercially available there.[1][5]
Uses
Promazine is a short-term add-on treatment for psychomotor agitation.[3]
Adverse effects
Common side effects include agitation, absent menstruation, arrhythmias, constipation, drowsiness and dizziness, dry mouth, problems with erection, tiredness, milky nipple discharge, large breasts, high sugars, difficulty sleeping, low blood pressure, prolonged QT, fits, shaking, vomiting and weight gain, among others.[3]
Overdose
In overdose, it may cause blood pressure to drop, lowering of body temperature, increased heart rate, and an irregular heart beat.[3]
Sudden death may occur, although rare.[3]
Other animals
Promazine, given as promazine hydrochloride, is one of the primary tranquilizers used by veterinarians as a pre-anaesthesia injection in horses.[6][7] It does not provide analgesia and is not a very strong sedative, hence it is used combined with opioids or α2 adrenoreceptor agonists, such as clonidine, or both.[7][8] It can be used alone when performing a non-painful procedure such as the fitting a horseshoe.[8] Low blood pressure, fast heart rate and paralysis of the penis are side effects.[6] It is also an antiemetic, antispasmodic and hypothermic agent.[7] Additionally it is used to lower blood pressure in animals with laminitis and kidney failure.[7] It is available in the US for veterinary use under the names Promazine and Tranquazine.
References
- "Promazine (Primazine, Prozine)". Davis's Drug Guide. Retrieved 6 August 2021.
- Davis C (2007). "Promazine". In Enna SJ, Bylund DB (eds.). X Pharm: The Comprehensive Pharmacology Reference. Elsevier. pp. 1–6. doi:10.1016/B978-008055232-3.62472-9. ISBN 978-0-08-055232-3.
- "BNF". NICE. Retrieved 4 August 2021.
- Pagliaro LA, Pagliaro AM (1999). "PPDR: Promazine". Psychologist's Neuropsychotropic Desk Reference. Philadelphia: Brunner/Mazel. p. 535. ISBN 978-1-138-00968-4.
- "Drugs@FDA: FDA-Approved Drugs". New Drug Application (NDA): 010942 Sparine. U.S. Food and Drug Administration.
- Hendrickson DA (2007). "2. Anaesthesia and fluid therapy". Techniques in Large Animal Surgery (3rd ed.). Blackwell Publishing. p. 16. ISBN 978-0-7817-8255-5.
- "Promazine". Equimed - Horse Health Matters. 20 January 2014. Archived from the original on 4 August 2021. Retrieved 4 August 2021.
- Ringer SK, Mama KR (2019). "23. Chemical restraint for standing procedures". In Auer JA, Stick JA (eds.). Equine Surgery (5th ed.). St Louis, Missouri: Elsevier. pp. 351–352. ISBN 978-0-323-48420-6.
- Wirth W (August 1958). "[The pharmacological action of promazine]". Arzneimittel-Forschung. 8 (8): 507–511. PMID 13584240.
- Szabo WA, Chung RH, Tam CC, Tishler M (February 1980). "Synthetic applications and mechanism of the pyrolysis of phenothiazine carbamates". The Journal of Organic Chemistry. 45 (4): 744–746. doi:10.1021/jo01292a048.
- Schmolka SJ, Zimmer H (1984). "N -Dimethylaminopropylation in a Solid-Liquid Two Phase System: Synthesis of Chlorpromazine, its Analogs, and Related Compounds". Synthesis. 1984 (1): 29–31. doi:10.1055/s-1984-30719.
- US 2519886, Charpentier P, issued 1950, assigned to Rhône-Poulenc.
- US 3100772, Kantor ML, Samuel T, issued 1963, assigned to Wyeth LLC.
- Dahl T, Tornøe CW, Bang-Andersen B, Nielsen P, Jørgensen M (15 February 2008). "Palladium-Catalyzed Three-Component Approach to Promazine with Formation of One Carbon–Sulfur and Two Carbon–Nitrogen Bonds". Angewandte Chemie International Edition. 47 (9): 1726–1728. doi:10.1002/anie.200705209.
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
| Classes |
|
|---|---|
| Antidepressants (TCAs and TeCAs) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Others |
|
