Levocetirizine
Levocetirizine, sold under the brand name Xyzal among others, is a second-generation antihistamine used for the treatment of allergic rhinitis (hay fever) and long term hives of unclear cause.[1] It is less sedating than older antihistamines.[2] It is taken by mouth.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Xyzal, Levazyr, others |
| Other names | Levocetirizine dihydrochloride |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607056 |
| License data | |
| Routes of administration | By mouth |
| Drug class | Second generation antihistamines |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | High |
| Protein binding | 90% |
| Metabolism | Liver 14% CYP3A4 |
| Elimination half-life | 6 to 10 hours |
| Excretion | Kidney and fecal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
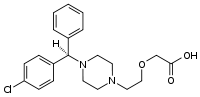
| Formula | C21H25ClN2O3 |
| Molar mass | 388.89 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Common side effects include sleepiness, dry mouth, cough, vomiting, and diarrhea.[1] Use in pregnancy appears safe but has not been well studied and use when breastfeeding is of unclear safety.[3] It is classified as a second-generation antihistamine and works by blocking histamine H1-receptors.[4][1]
Levocetirizine was approved for medical use in the United States in 2007.[1] It is available as a generic medication.[2] In 2019, it was the 193rd most commonly prescribed medication in the United States, with more than 2 million prescriptions.[5][6]
Medical uses
Levocetirizine is used for allergic rhinitis.[7] This includes allergy symptoms such as watery eyes, runny nose, sneezing, hives, and itching.[8]
Side effects
Levocetirizine is referred to as a non-sedating antihistamine as it does not enter the brain in significant amounts and is therefore unlikely to cause drowsiness. Cardiac safety with repolarization may be better than some other antihistamines, as levocetirizine does not significantly prolong the QT interval in healthy individuals.[9][10][11] However, some people may still experience some slight sleepiness, headache, mouth dryness, lightheadedness, vision problems (mainly blurred vision), palpitations and fatigue.[12]
Pharmacology
Levocetirizine is an antihistamine. It acts as an inverse agonist that decreases activity at histamine H1 receptors. This in turn prevents the release of other allergy chemicals and increases the blood supply to the area, providing relief from the typical symptoms of hay fever. Levocetirizine, (R)-(-)-cetirizine, is essentially a chiral switch of (±)-cetirizine. This enantiomer, the eutomer, is more selective and less sedative and the (S)-counterpart, the distomer, is inactive.[13][14]
Chemistry
Chemically, levocetirizine is the active levorotary enantiomer of cetirizine, also called the l-enantiomer of cetirizine. It is a member of the diphenylmethylpiperazine group of antihistamines.
History
Levocetirizine was first launched in 2001 by the Belgian pharmaceutical company UCB (Union Chimique Belge).
Society and culture
Availability
On 31 January 2017, the Food and Drug Administration approved an over-the-counter preparation.[15] Levocetirizine had previously received authorization by the FDA as a prescription drug in 2007, having already been brought to market throughout much of Europe. In India, a prescription-only drug containing levocetirizine hydrochloride and montelukast is sold as Crohist MK.
Brand names
Preparations of levocetirizine are sold under the following brand names:
- Xyzal /ˈzaɪˌzɑːl/ in Australia, Austria, Bulgaria, Croatia, Cyprus, Czech Republic, Finland, France, Hungary, India, Ireland (also Rinozal), Italy, Japan, Lithuania, Netherlands, Poland, Portugal, Romania, Taiwan, Thailand, Turkey, The Philippines, Serbia, Singapore, Slovakia, Slovenia, South Africa, Spain, Switzerland and UK. On May 25, 2007, the United States Food and Drug Administration approved Xyzal, where it is co-marketed by Sanofi-Aventis.
- Zobral in Cyprus
- Levobert in India
- Xusal in Germany and Mexico
- Xozal in Greece
- Degraler in Chile
- Allevo in Egypt
- Zilola, Histisynt, and Xyzal (UCB) in Hungary
- Alcet, Curin, and Seasonix in Bangladesh
- Vozet and Uvnil in India
- T-Day Syrup in Pakistan
- Curin in Nepal.[16]
- Zenaro in the Czech Republic and Slovakia
- Xuzal and Zival in Chile
- Cezera, Levosetil, Robenan, and Xyzal in Serbia.[17]
- Rinozal and Xyzal in Ireland
References
- "Levocetirizine Dihydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 22 March 2019.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 280–281. ISBN 9780857113382.
- "Levocetirizine Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, et al. (August 2008). "The diagnosis and management of rhinitis: an updated practice parameter". The Journal of Allergy and Clinical Immunology. 122 (2 Suppl): S1-84. doi:10.1016/j.jaci.2008.06.003. PMID 18662584.
- "The Top 300 of 2019". ClinCalc. Retrieved 16 October 2021.
- "Levocetirizine - Drug Usage Statistics". ClinCalc. Retrieved 16 October 2021.
- Holgate S, Powell R, Jenkins M, Ali O (July 2005). "A treatment for allergic rhinitis: a view on the role of levocetirizine". Current Medical Research and Opinion. Informa Healthcare. 21 (7): 1099–1106. doi:10.1185/030079905x53298. PMID 16004679. S2CID 26620889.
The variable efficacy and durability of response of different antihistamines arise from differing modulatory effects on the H(1)-receptor. Conclusion: These findings support both the short-term and long-term use of levocetirizine in the clinical management of allergic rhinitis. The World Health Organization (WHO) ARIA Guidelines (Allergic Rhinitis and its Impact on Asthma), recommend using a combination of a non-sedating antihistamine with a decongestant, or glucocorticosteroids for treating allergic rhinitis - with the order and combination of treatment depending on severity and duration of symptoms.
- "Levocetirizine Oral". WebMD.
- Hulhoven R, Rosillon D, Letiexhe M, Meeus MA, Daoust A, Stockis A (November 2007). "Levocetirizine does not prolong the QT/QTc interval in healthy subjects: results from a thorough QT study". European Journal of Clinical Pharmacology. 63 (11): 1011–1017. doi:10.1007/s00228-007-0366-5. PMID 17891537. S2CID 36218027.
- "Cetirizine and loratadine: minimal risk of QT prolongation". Prescrire International. 19 (105): 26–28. February 2010. PMID 20455340.
- Poluzzi E, Raschi E, Godman B, Koci A, Moretti U, Kalaba M, et al. (2015). "Pro-arrhythmic potential of oral antihistamines (H1): combining adverse event reports with drug utilization data across Europe". PLOS ONE. 10 (3): e0119551. Bibcode:2015PLoSO..1019551P. doi:10.1371/journal.pone.0119551. PMC 4364720. PMID 25785934.
- XOZAL technical specifications booklet.
- Wang DY, Hanotte F, De Vos C, Clement P (April 2001). "Effect of cetirizine, levocetirizine, and dextrocetirizine on histamine-induced nasal response in healthy adult volunteers". Allergy. 56 (4): 339–343. doi:10.1034/j.1398-9995.2001.00775.x. PMID 11284803. S2CID 11304832.
- Devalia JL, De Vos C, Hanotte F, Baltes E (January 2001). "A randomized, double-blind, crossover comparison among cetirizine, levocetirizine, and ucb 28557 on histamine-induced cutaneous responses in healthy adult volunteers". Allergy. 56 (1): 50–57. doi:10.1034/j.1398-9995.2001.00726.x. PMID 11167352. S2CID 40716352.
- "Prescription to Over-the-Counter (OTC) Switch List". Food and Drug Administration. Retrieved February 9, 2017.
- "Curin". Archived from the original on October 22, 2012. Retrieved August 29, 2012.
- "Medicines and Medical Devices Agency of Serbia". Retrieved August 23, 2020.
External links
- "Levocetirizine". Drug Information Portal. U.S. National Library of Medicine.
- "Levocetirizine dihydrochloride". Drug Information Portal. U.S. National Library of Medicine.