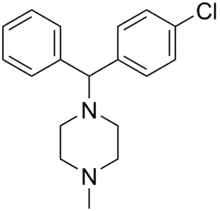
Chlorcyclizine
Chlorcyclizine (Di-Paralene, Mantadil, Pruresidine, Trihistan) is a first-generation antihistamine of the diphenylmethylpiperazine group marketed in the United States and certain other countries.[1][2][3] It is used primarily to treat allergy symptoms such as rhinitis, urticaria, and pruritus, and may also be used as an antiemetic.[1][2][3] In addition to its antihistamine effects, chlorcyclizine also has some anticholinergic, antiserotonergic, and local anesthetic properties.[4][5] It also has been studied as a potential treatment for various flaviviruses like Hepatitis C and Zika Virus.[6][7][8]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a682619 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.315 |
| Chemical and physical data | |
| Formula | C18H21ClN2 |
| Molar mass | 300.83 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
See also
References
- Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- David J. Triggle (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. ISBN 0-412-46630-9.
- Hall, Judith A.; Morton, Ian (1999). Concise dictionary of pharmacological agents: properties and synonyms. Kluwer Academic. ISBN 0-7514-0499-3.
- Dorland Staff (2008). Dorland Dictionnaire Medical Bilingue Francais-anglais / Anglais-francais: + E-book a Telecharger (French ed.). Elsevier (Educa Books). ISBN 978-2-84299-899-8.
- Rogóz Z, Skuza G, Sowińska H (November 1981). "The effect of the antihistaminic drugs on the central action of 5-hydroxytryptophan in mice". Polish Journal of Pharmacology and Pharmacy. 33 (4): 459–65. PMID 6120505.
- He, S.; Lin, B.; Chu, V.; Hu, Z.; Hu, X.; Xiao, J.; Wang, A. Q.; Schweitzer, C. J.; Li, Q.; Imamura, M.; Hiraga, N.; Southall, N.; Ferrer, M.; Zheng, W.; Chayama, K.; Marugan, J. J.; Liang, T. J. (2015). "Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection". Science Translational Medicine. 7 (282): 282ra49. doi:10.1126/scitranslmed.3010286. PMC 6420960. PMID 25855495.
- Chamoun-Emanuelli, Ana Maria; Pecheur, Eve-Isabelle; Chen, Zhilei (July 2014). "Benzhydrylpiperazine compounds inhibit cholesterol-dependent cellular entry of hepatitis C virus". Antiviral Research. 109: 141–148. doi:10.1016/j.antiviral.2014.06.014. PMID 25019406.
- Santos, Felipe R. S.; Nunes, Damiana A. F.; Lima, William G.; Davyt, Danilo; Santos, Luciana L.; Taranto, Alex G.; M. S. Ferreira, Jaqueline (2019). "Identification of Zika Virus NS2B-NS3 Protease Inhibitors by Structure-Based Virtual Screening and Drug Repurposing Approaches". Journal of Chemical Information and Modeling. 60 (2): 731–737. doi:10.1021/acs.jcim.9b00933. ISSN 1549-9596. PMID 31850756.
| Benzimidazoles (*) | |
|---|---|
| Diarylmethanes |
|
| Ethylenediamines | |
| Tricyclics | |
| Others |
|
| For topical use | |
Acetylcholine receptor modulators | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
Histamine receptor modulators | |||||
|---|---|---|---|---|---|
| H1 |
| ||||
| H2 |
| ||||
| H3 |
| ||||
| H4 |
| ||||
| |||||
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Simple piperazines (no additional rings) |
|
|---|---|
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines |
|
| Pyridinylpiperazines |
|
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.