Anileridine
Anileridine (trade name: Leritine) is a synthetic analgesic drug[1] and is a member of the piperidine class of analgesic agents[2] developed by Merck & Co. in the 1950s.[3] It differs from pethidine (meperidine) in that the N-methyl group of meperidine is replaced by an N-aminophenethyl group, which increases its analgesic activity.
 | |
| Clinical data | |
|---|---|
| Trade names | Leritine |
| AHFS/Drugs.com | Monograph |
| Routes of administration | Tablets, injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | > 95% |
| Metabolism | Hepatic |
| Identifiers | |
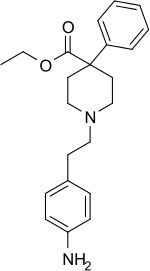
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H28N2O2 |
| Molar mass | 352.478 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 83 °C (181 °F) |
SMILES
| |
InChI
| |
| | |
Anileridine is no longer manufactured in the US or Canada.[4] Anileridine is in Schedule II of the Controlled Substances Act 1970 of the United States as ACSCN 9020 with a zero aggregate manufacturing quota as of 2014. The free base conversion ratio for salts includes 0.83 for the dihydrochloride and 0.73 for the phosphate.[5] It is also under international control per UN treaties.
Pharmacokinetics
Anileridine usually takes effect within 15 minutes of either oral or intravenous administration, and lasts 2–3 hours.[7] It is mostly metabolized by the liver.
References
- Orahovats PD, Lehman EG, Chapin EW (January 1957). "Pharmacology of ethyl-1-(4-aminophenethyl)-4-phenylisonipecotate, anileridine, a new potent synthetic analgesic". The Journal of Pharmacology and Experimental Therapeutics. 119 (1): 26–34. PMID 13417056.
- Stage JT (August 1957). "Anileridine as an anesthetic agent". The Journal of the Florida Medical Association. Florida Medical Association. 44 (2): 143–5. PMID 13449255.
- US 2897204, Frank A Cutler Jr FA, Chemerda JM, "Substituted piperidines and methods for making same", issued 28 July 1959, assigned to Merck and Co Inc
- "Discontinued Prescription Drug Products". Canadian Pharmacists' Association. Archived from the original on 19 September 2008. Retrieved 28 July 2008.
- "Federal Register Notices: Quotas - 2014". Diversion Control Division, Drug Enforcement Agency. U.S. Department of Justice. 25 August 2014. Archived from the original on 4 March 2016. Retrieved 26 February 2016.
- "Pharmaceutical Information - LERITINE". RxMed. Retrieved 16 June 2010.
- "Anileridine Consumer Information". MedicineNet. Archived from the original on 28 March 2012. Retrieved 28 July 2008.