Zomepirac
Zomepirac is an orally effective nonsteroidal anti-inflammatory drug (NSAID) that has antipyretic actions. It was developed by McNeil Pharmaceutical, approved by the FDA in 1980, and sold as the sodium salt zomepirac sodium, under the brand name Zomax. Due to its clinical effectiveness, it was preferred by doctors in many situations and obtained a large share of the analgesics market; however, it was subsequently withdrawn in March 1983 due to its tendency to cause serious anaphylaxis in a small, but unpredictable, subset of the patient population.[1][2]
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.780 |
| Chemical and physical data | |
| Formula | C15H14ClNO3 |
| Molar mass | 291.73 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Indications
Zomepirac was indicated for the management of mild to severe pain.[3] Multiple clinical trials demonstrated zomepirac to be more effective than aspirin or codeine alone and to be as effective as analgesic combinations containing codeine or other opioids.[4][5][6][7][8][9][10] Zomepirac provided analgesia comparable with usual intramuscular doses of morphine in postoperative pain and that with long-term use, neither tolerance to its analgesic effect nor psychological or physical dependence had been demonstrated.[3][11]
Chemical structure
Zomepirac is the sodium salt of 5-(4-chlorobenzoyl)-1,4 dimethyl-1H-pyrrole-2-acetate dihydrate. It is a pyrrole-acetic acid which is structurally related to tolmetin. The chemical structure differs from other NSAIDs in that the central benzene ring has been replaced by a pyrrole.
Mechanism of action
Zomepirac is a prostaglandin synthetase inhibitor.[12]
Anaphylaxis
Zomepirac does not cause anaphylaxis directly, but it is metabolized by UDP-glucuronosyltransferase (UGT) to a reactive glucuronide which binds irreversibly to plasma albumin.[13]
Synthesis
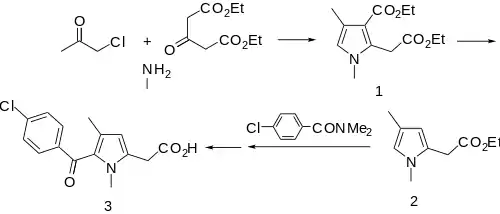
Zomepirac can be synthesized from diethyl 1,3-acetonedicarboxylate, chloroacetone, and aqueous methylamine (MeNH2) via modification of the Hantzsch pyrrole synthesis to give intermediate 1. Saponification, monoesterification, and thermal decarboxylation gives ester 2. This is acylated with N,N-dimethyl-p-chlorobenzamide, and finally saponification gives zomepirac (3).
See also
References
- Peter H. Rheinstein (September 1992). "Reporting of adverse drug events: a key to postmarketing drug safety". American Family Physician.
- Mark P. Grillo and Fengmei Hua (August 19, 2003). "Identification of Zomepirac-S-Acyl-Glutathione in In Vitro Incubations with Rat Hepatocyles and In Vitro Rate Bile". Drug Metabolism and Disposition. 31 (11): 1429–1436. doi:10.1124/dmd.31.11.1429. PMID 14570776. S2CID 9912756.
- Lewis JR (1981). "Zomepirac sodium. A new nonaddicting analgesic". JAMA. 246 (4): 377–9. doi:10.1001/jama.246.4.377. PMID 7241789.
- Steele CE, Jefferson WL (1983). "A multi-centre study of zomepirac in painful conditions: an analysis of clinical data for 15,484 patients". Current Medical Research and Opinion. 8 (6): 382–91. doi:10.1185/03007998309111743. PMID 6221886.
- Mehlisch DR, Joy ED (1981). "Zomepirac sodium vs APC with codeine for oral surgery pain". Journal of Oral Surgery. 39 (6): 426–9. PMID 7014804.
- Stambaugh JE, Sarajian C (1981). "Analgesic efficacy of zomepirac sodium in patients with pain due to cancer". Journal of Clinical Pharmacology. 21 (11–12 Pt 1): 501–7. doi:10.1002/j.1552-4604.1981.tb05657.x. PMID 7037868. S2CID 19347859.
- Evans PJ, McQuay HJ, Rolfe M, O'Sullivan G, Bullingham RE, Moore RA (1982). "Zomepirac, placebo and paracetamol/dextropropoxyphene combination compared in orthopaedic postoperative pain". British Journal of Anaesthesia. 54 (9): 927–33. doi:10.1093/bja/54.9.927. PMID 7052110.
- Baird WM, Turek D (1980). "Comparison of zomepirac, APC with codeine, codeine and placebo in the treatment of moderate and severe postoperative pain". Journal of Clinical Pharmacology. 20 (4 Pt 2): 243–9. doi:10.1002/j.1552-4604.1980.tb01704.x. PMID 6991540. S2CID 7637029.
- Mehlisch DR, Joy ED, Moore TE, Porter K, Stumpf AJ, Wolfe SH (1980). "Clinical comparison of zomepirac with APC/codeine combination in the treatment of pain following oral surgery". Journal of Clinical Pharmacology. 20 (4 Pt 2): 271–8. doi:10.1002/j.1552-4604.1980.tb01708.x. PMID 6991544. S2CID 45366160.
- Diamond S, Medina JL (1981). "A double-blind study of zomepirac sodium and placebo in the treatment of muscle contraction headache". Headache. 21 (2): 45–8. doi:10.1111/j.1526-4610.1981.hed2102045.x. PMID 7016809. S2CID 41806718.
- Wallenstein SL, Rogers A, Kaiko RF, Heidrich G, Houde RW (1980). "Relative analgesic potency of oral zomepirac and intramuscular morphine in cancer patients with postoperative pain". Journal of Clinical Pharmacology. 20 (4 Pt 2): 250–8. doi:10.1002/j.1552-4604.1980.tb01705.x. PMID 6991541. S2CID 11808742.
- DC McLeod, Zomepirac (Zomax, McNeil Pharmaceutical) Archived September 28, 2007, at the Wayback Machine, Drug Intelligence & Clinical Pharmacy: Vol. 15, No. 7, pp. 522-530.
- Smith, P. C.; McDonagh, A. F.; Benet, L. Z. (1986). "Irreversible binding of zomepirac to plasma protein in vitro and in vivo". Journal of Clinical Investigation. 77 (3): 934–939. doi:10.1172/JCI112392. PMC 423485. PMID 3949982.
- Carson, John R.; Wong, Stewart (1973). "5-Benzoyl-1-methylpyrrole-2-acetic acids as antiinflammatory agents. 2. 4-Methyl compounds". Journal of Medicinal Chemistry. 16 (2): 172–4. doi:10.1021/jm00260a023. PMID 4683116.
- DE 2102746, Carson, John Robert, "5-Aroylpyrrole [5-aroyl pyrroles]", published 1971-08-12, assigned to McNeil Laboratories Inc.
- J. R. Carson, U.S. Patent 3,752,826 (1973 to McNeil).