Flurbiprofen
Flurbiprofen is a member of the phenylalkanoic acid derivative family of nonsteroidal anti-inflammatory drugs (NSAIDs). It is primarily indicated as a pre-operative anti-miotic (in an ophthalmic solution) as well as orally for arthritis or dental pain. Side effects are analogous to those of ibuprofen.[1]
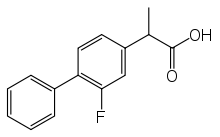 | |
| Clinical data | |
|---|---|
| Trade names | Ansaid, Ocufen, Strepfen |
| Other names | (±)-2-fluoro-α-methyl-(1,1'-biphenyl)-4-acetic acid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a687005 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | > 99% |
| Metabolism | Liver (CYP2C9) |
| Elimination half-life | 4.7-5.7 hours |
| Excretion | Kidney |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.023.479 |
| Chemical and physical data | |
| Formula | C15H13FO2 |
| Molar mass | 244.265 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 117 °C (243 °F) |
SMILES
| |
InChI
| |
| (verify) | |
It was derived from propionic acid by the research arm of Boots UK during the 1960s, a period which also included the discovery of ibuprofen, indometacin, diclofenac, naproxen, ketoprofen, and sulindac.[2][3]: 34
It was patented in 1964 by Boots UK and approved for medical use in 1987.[4] It was approved in the US in 1988; the first generic was approved in 1994.[5]: 158
Adverse effects
In October 2020, the U.S. Food and Drug Administration (FDA) required the drug label to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of kidney problems in unborn babies that result in low amniotic fluid.[6][7] They recommend avoiding NSAIDs in pregnant women at 20 weeks or later in pregnancy.[6][7]
Society and culture
Brand names
As of 2016 the drug was available worldwide as drops for ophthalmic use and as tablets, both in various strengths, under many brand names which include: Acustop Cataplasma, Adofeed, Anazin, Anflupin, Anorcid, Ansaid, Antadys, Antafen, Antipain, Baenazin, Benactiv, Biprofin, Biprotec, Bro-Z, Brufen, Brufoz, Cebutid, Clinadol, Coryfin, Dispain, Edolfene, Eyeflur, Falken, Fiera, Flu Ro Fen, Flubifix, Flufen, Flugalin, Flupe, Flur di fen, Fluractive, Fluran, Flurbi Pap, Flurbic, Flurbiprofen, Flurbiprofène, Flurbiprofeno, Flurflex, Flurofen, Fluroptic, Fo Bi Pu Luo Fun, Forphen, Fortine, Froben, Frolix, Fubifen, Fubiprofen, Fubofen, Fukon, Fulruban, Furofen, Kai Fen, Kavoflog, Kotton, Lefenine, Majezik, Maprofen, Maxaljin, Maximus, Meiprofen, Neliacan, Nibelon, Nirolex Gola, Ocufen, Ocuflur, Optifen, Orofaringeo, Painil, Profen, Projezik, Ropion, Sigmaprofen, Stayban, Strefen, Strepfen, Strepflam, Strepsils (various formulations), Sulan, Tie Shr Shu, TransAct, Upnon, Urbifen, Yakuban, Zepolas, Zeralgo, Zero-P, and Zeton.[8]
Synthesis
Patent quotation: "It has anti-inflammatory activity which is about 240 times that of aspirin and analgesic activity which is about 180 times that of aspirin in standard laboratory tests."
The Malonic ester synthesis between 2,4-Difluoronitrobenzene [446-35-5] (1) and Diethyl methylmalonate [609-08-5] (2) gives Diethyl (3-fluoro-4-nitrophenyl)methylmalonate [78543-06-3] (3). The catalytic hydrogenation of the nitro group gives Diethyl (4-amino-3-fluorophenyl)methylmalonate [78543-08-5] (4). Sandmeyer reaction of the diazonium salt with benzene (5) apparently gives CID:13065233 (6). Saponification of the esters gives 2-(2-Fluorobiphenyl-4-yl)-2,3-dimethylbutanedioic acid [42771-82-4] (7). Boiling in acid causes decarboxylation, completing the synthesis of Flurbiprofen (8).
References
- "Lexicomp: Flurbiprofen". Lexicomp. Wolters Kluwer. Retrieved 25 September 2015.
- Rainsford KD (December 2011). "Fifty years since the discovery of ibuprofen". Inflammopharmacology. 19 (6): 293–7. doi:10.1007/s10787-011-0103-7. PMID 22120888.
- Fischer J, Ganellin CR (2010). Analogue-based Drug Discovery II. John Wiley & Sons. ISBN 9783527632121.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 520. ISBN 9783527607495.
- Approved Drug Products with Therapeutic Equivalence Evaluations (PDF) (36th ed.). FDA. 2014.
- "FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications". U.S. Food and Drug Administration (FDA) (Press release). 15 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "NSAIDs may cause rare kidney problems in unborn babies". U.S. Food and Drug Administration. 21 July 2017. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Flurbiprofen - International Brand Names". Drugs.com. Retrieved 14 November 2016.
- Deshmukh, M. N.; Lakshminarayana, V. (1998). "IMPROVED PREPARATION OF FLURBIPROFEN". Organic Preparations and Procedures International. 30 (4): 453–455. doi:10.1080/00304949809355309.
- Jerry A. Walker, U.S. Patent 4,266,069 (1981 to Pharmacia and Upjohn Co).
Further reading
- Dean L (2019). "Flurbiprofen Therapy and CYP2C9 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 30742399. Bookshelf ID: NBK537365.
.svg.png.webp)