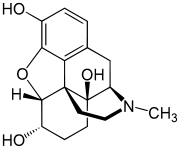
Hydromorphinol
Hydromorphinol (RAM-320, 14-Hydroxydihydromorphine),[1] also is an opiate analogue that is a derivative of morphine, where the 14-position has been hydroxylated and the 7,8- double bond saturated.[2] It has similar effects to morphine such as sedation, analgesia and respiratory depression, but is twice as potent as morphine[1] and has a steeper dose-response curve and longer half-life.[3] It is used in medicine as the bitartrate salt (free base conversion ratio 0.643, molecular weight 471.5) and hydrochloride (free base conversion ratio 0.770, molecular weight 393.9)
 | |
| Clinical data | |
|---|---|
| Other names | Hydromorphinol, 14-hydroxy-7,8-dihydromorphine, RAM-320 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.878 |
| Chemical and physical data | |
| Formula | C17H21NO4 |
| Molar mass | 303.358 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
It is also called α-Oxymorphol, and oxymorphol is itself a mixture of hydromorphinol and 4,5α-Epoxy-17-methylmorphinan-3,6β,14-triol, β-Oxymorphol, which is different at position 6 on the morphine carbon skeleton.[4]
Hydromorphinol was developed in Austria in 1932. In the United States, it was never available and is classified as a Schedule I drug with a DEA ACSCN of 9301. The salts in use are the bitartrate (free base conversion ratio 0.643) and hydrochloride (0.770). The 2014 national aggregate manufacturing quota was 2 grams, unchanged from prior years.[5]
Hydromorphinol is metabolised mainly in the liver in the same fashion as many other opioids and is itself a minor active metabolite of 14-Hydroxydihydrocodeine, an uncommonly used opiate (but is therefore also an active metabolite of a first-order active metabolite of oxycodone).
It is distributed under the trade name Numorphan in some countries. It is controlled under the Single Convention On Narcotic Drugs.
See also
- Oxymorphol
- N-Phenethylhydromorphinol (RAM-378)
References
- US 2960505, Weiss U, "Morphine derivative", published 11/15/1960
- Weiss U, Daum SJ (January 1965). "Derivatives of Morphine. IV.114-Hydroxymorphine and 14-Hydroxydihydromorphine". Journal of Medicinal Chemistry. 8: 123–5. doi:10.1021/jm00325a028. PMID 14287245.
- Plummer JL, Cmielewski PL, Reynolds GD, Gourlay GK, Cherry DA (March 1990). "Influence of polarity on dose-response relationships of intrathecal opioids in rats". Pain. 40 (3): 339–47. doi:10.1016/0304-3959(90)91131-2. PMID 2326098. S2CID 2100412.
- "Hydromorphinol CAS#: 2183-56-4". Chemical Book.
- "Quotas 2014". DEA Diversion Control Division. Archived from the original on 2016-03-04. Retrieved 2016-02-27.