Tabernanthine
Tabernanthine is an alkaloid found in Tabernanthe iboga.[1]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
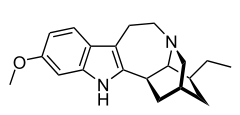
| Formula | C20H26N2O |
| Molar mass | 310.441 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
It has been used in laboratory experiments to study how addiction affects the brain.[2]
Tabernanthine persistently reduced the self-administration of cocaine and morphine in rats.[3]
Pharmacology
It is kappa opioid agonist (Ki = 0.15 μM) and NMDA receptor (Ki = 10.5 μM) antagonist.[4][5] Compared to ibogaine, it binds weakly to σ1 and σ2 receptor.[5]
See also
- Coronaridine
- Ibogamine
- Voacangine
- Tabernaemontanine
- Tabernanthalog
References
- Bartlett, M. F.; Dickel, D. F.; Taylor, W. I. (1958). "The Alkaloids of Tabernanthe iboga. Part IV.1 The Structures of Ibogamine, Ibogaine, Tabernanthine and Voacangine". Journal of the American Chemical Society. 80: 126–136. doi:10.1021/ja01534a036.
- Levi MS, Borne RF (October 2002). "A review of chemical agents in the pharmacotherapy of addiction". Curr. Med. Chem. 9 (20): 1807–18. doi:10.2174/0929867023368980. PMID 12369879.
- Glick SD, Kuehne ME, Raucci J, Wilson TE, Larson D, Keller RW Jr, Carlson JN (September 1994). "Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum". Brain Res. 657 (1–2): 14–22. doi:10.1016/0006-8993(94)90948-2. PMID 7820611. S2CID 1940631.
- Deecher DC, Teitler M, Soderlund DM, Bornmann WG, Kuehne ME, Glick SD (February 1992). "Mechanisms of action of ibogaine and harmaline congeners based on radioligand binding studies". Brain Research. 571 (2): 242–7. doi:10.1016/0006-8993(92)90661-r. PMID 1377086. S2CID 17159661.
- Christophe Wiart (16 December 2013). Lead Compounds from Medicinal Plants for the Treatment of Neurodegenerative Diseases. Academic Press. pp. 67–69, 73. ISBN 978-0-12-398383-1.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.