Piracetam
Piracetam is a drug marketed as a treatment for myoclonus.[3] It is also used as a cognitive enhancer to improve memory, attention, and learning.[4][5][6][7][8] Evidence to support its use is unclear, with some studies showing modest benefits in specific populations and others showing minimal or no benefit.[9][10] Piracetam is sold as a medication in many European countries. Sale of piracetam is not illegal in the United States, although it is not regulated nor approved by the FDA so it is legally sold for research use only.[4]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Breinox, Dinagen, Lucetam, Nootropil, Nootropyl, Oikamid, Piracetam and many others |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth, parenteral, or vaporized |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Onset of action | Swiftly following administration. Food delays time to peak concentration by 1.5 h approximately to 2–3 h since dosing.[2] |
| Elimination half-life | 4–5 h |
| Excretion | Urinary |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.028.466 |
| Chemical and physical data | |
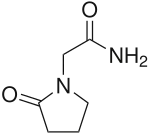
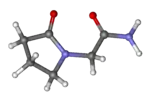
| Formula | C6H10N2O2 |
| Molar mass | 142.158 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 152 °C (306 °F) |
SMILES
| |
InChI
| |
| (verify) | |
Piracetam is in the racetams group, with chemical name 2-oxo-1-pyrrolidine acetamide. It is a derivative of the neurotransmitter GABA[9] and shares the same 2-oxo-pyrrolidone base structure with pyroglutamic acid. Piracetam is a cyclic derivative of GABA (gamma-aminobutyric acid). Related drugs include the anticonvulsants levetiracetam and brivaracetam, and the putative nootropics aniracetam and phenylpiracetam.
Efficacy
Dementia
A 2001 Cochrane review concluded that there was not enough evidence to support piracetam for dementia or cognitive problems.[10] A 2005 review found some evidence of benefit in older subjects with cognitive impairment.[9] In 2008, a working group of the British Academy of Medical Sciences noted that many of the trials of piracetam for dementia were flawed.[11]
There is no good evidence that piracetam is of benefit in treating vascular dementia.[12]
Depression and anxiety
Some sources suggest that piracetam's overall effect on lowering depression and anxiety is higher than on improving memory.[13] However, depression is reported to be an occasional adverse effect of piracetam.[14]
Other
Piracetam may facilitate the deformability of erythrocytes in capillary which is useful for cardiovascular disease.[9][3]
Peripheral vascular effects of piracetam have suggested its use potential for vertigo, dyslexia, Raynaud's phenomenon and sickle cell anemia.[9][3] There is no evidence to support piracetam's use in sickle cell crisis prevention[15] or for fetal distress during childbirth.[16] There is no evidence for benefit of piracetam with acute ischemic stroke,[17] though there is debate as to its utility during stroke rehabilitation.[18][19]
Anti-vasospasm
Piracetam has been found to diminish erythrocyte adhesion to vascular wall endothelium, making any vasospasm in the capillary less severe. This contributes to its efficacy in promoting microcirculation, including to the brain and kidneys.[9][3]
Side effects
Symptoms of general excitability, including anxiety, insomnia, irritability, headache, agitation, nervousness, tremor, and hyperkinesia, are occasionally reported.[14][20][21] Other reported side effects include somnolence, weight gain, clinical depression, weakness, increased libido, and hypersexuality.[14]
According to a 2005 review, piracetam has been observed to have the following side effects: hyperkinesia, weight gain, nervousness, somnolence, depression and asthenia.[9]
Piracetam reduces platelet aggregation as well as fibrinogen concentration, and thus is contraindicated to patients with cerebral hemorrhage.[9][3]
Toxicity
The LD50 for oral consumption in humans has not been determined.[22] The LD50 is 5.6 g/kg for rats and 20 g/kg for mice, indicating extremely low acute toxicity.[23] For comparison, in rats the LD50 of vitamin C is 12 g/kg and the LD50 of table salt is 3 g/kg.
Mechanisms of action
Piracetam's mechanism of action, as with racetams in general, is not fully understood. The drug influences neuronal and vascular functions and influences cognitive function without acting as a sedative or stimulant.[9] Piracetam is a positive allosteric modulator of the AMPA receptor, although this action is very weak and its clinical effects may not necessarily be mediated by this action.[24] It is hypothesized to act on ion channels or ion carriers, thus leading to increased neuron excitability.[22] GABA brain metabolism and GABA receptors are not affected by piracetam[25]
Piracetam improves the function of the neurotransmitter acetylcholine via muscarinic cholinergic (ACh) receptors, which are implicated in memory processes.[26] Furthermore, piracetam may have an effect on NMDA glutamate receptors, which are involved with learning and memory processes. Piracetam is thought to increase cell membrane permeability.[26][27] Piracetam may exert its global effect on brain neurotransmission via modulation of ion channels (i.e., Na+, K+).[22] It has been found to increase oxygen consumption in the brain, apparently in connection to ATP metabolism, and increases the activity of adenylate kinase in rat brains.[28][29] Piracetam, while in the brain, appears to increase the synthesis of cytochrome b5,[30] which is a part of the electron transport mechanism in mitochondria. But in the brain, it also increases the permeability of some intermediates of the Krebs cycle through the mitochondrial outer membrane.[28]
Piracetam inhibits N-type calcium channels. The concentration of piracetam achieved in central nervous system after a typical dose of 1200 mg (about 100 μM)[31] is much higher than the concentration necessary to inhibit N-type calcium channels (IC50 of piracetam in rat neurons was 3 μM).[32]
History
Piracetam was first made some time between the 1950s and 1964 by Corneliu E. Giurgea.[33] There are reports of it being used for epilepsy in the 1950s.[34]
Society and culture
In 2009 piracetam was reportedly popular as a cognitive enhancement drug among students.[35]
Legal status
Piracetam is an uncontrolled substance in the United States meaning it is legal to possess without a license or prescription.[36]
Regulatory status
In the United States, piracetam is not approved by the Food and Drug Administration.[1] Piracetam is not permitted in compounded drugs or dietary supplements in the United States.[37] Like most research chemicals, it has been available over-the-counter, self-regulated, and third-party lab tested by many US companies for decades.[4]
In the United Kingdom, piracetam is approved as a prescription drug Prescription Only Medicine (POM) number is PL 20636/2524[38] for adult with myoclonus of cortical origin, irrespective of cause, and should be used in combination with other anti-myoclonic therapies.[39]
In Japan piracetam is approved as a prescription drug.[40]
Piracetam has no DIN in Canada, and thus cannot be sold but can be imported for personal use in Canada.[41]
In Hungary, piracetam was a prescription-only medication, but as of 2020, no prescription is required and piracetam is available as an over-the-counter drug under the name Memoril Mite, and is available in 600 mg pills.
See also
- AMPA receptor positive allosteric modulator
- Aniracetam
- Brivaracetam—an analogue of piracetam with the same additional side chain as levetiracetam and a three–carbon chain. It exhibits greater antiepileptic properties than levetiracetam in animal models, but with a somewhat smaller, although still high, therapeutic range.
- Hydergine
- Levetiracetam—an analogue of piracetam bearing an additional CH3–CH2– sidechain and bearing antiepileptic pharmacological properties through a poorly understood mechanism probably related to its affinity for the vesicle protein SV2A.
- Oxiracetam
- Phenylpiracetam—a phenylated analog of the drug piracetam which was developed in 1983 in Russia where it is available as a prescription drug.
- Pramiracetam
References
- "Piracetam". DrugBank database.
- Leaflet of Piracetam.
- "Nootropil Tablets 1200 mg". (emc). 15 February 2017. Retrieved 14 April 2019.
- Cohen PA, Zakharevich I, Gerona R (March 2020). "Presence of Piracetam in Cognitive Enhancement Dietary Supplements". JAMA Internal Medicine. 180 (3): 458–459. doi:10.1001/jamainternmed.2019.5507. PMC 6902196. PMID 31764936.
- Müller WE, Koch S, Scheuer K, Rostock A, Bartsch R (January 1997). "Effects of piracetam on membrane fluidity in the aged mouse, rat, and human brain". Biochemical Pharmacology. 53 (2): 135–140. doi:10.1016/s0006-2952(96)00463-7. PMID 9037245.
- Zhu D, Bungart BL, Yang X, Zhumadilov Z, Lee JC, Askarova S (2015). "Role of membrane biophysics in Alzheimer's-related cell pathways". Frontiers in Neuroscience. 9: 186. doi:10.3389/fnins.2015.00186. PMC 4444756. PMID 26074758.
- Dimond SJ, Brouwers EM (September 1976). "Increase in the power of human memory in normal man through the use of drugs". Psychopharmacology. 49 (3): 307–309. doi:10.1007/BF00426834. PMID 826948. S2CID 43958165.
- Mindus P, Cronholm B, Levander SE, Schalling D (August 1976). "Piracetam-induced improvement of mental performance. A controlled study on normally aging individuals". Acta Psychiatrica Scandinavica. 54 (2): 150–160. doi:10.1111/j.1600-0447.1976.tb00107.x. PMID 785952. S2CID 39067958.
- Winblad B (2005). "Piracetam: a review of pharmacological properties and clinical uses". CNS Drug Reviews. 11 (2): 169–182. doi:10.1111/j.1527-3458.2005.tb00268.x. PMC 6741724. PMID 16007238.
- Flicker L, Grimley Evans G (2001). "Piracetam for dementia or cognitive impairment". The Cochrane Database of Systematic Reviews (2): CD001011. doi:10.1002/14651858.CD001011. PMID 11405971.
- Horne G, et al. (May 2008). Brain science, addiction and drugs (PDF) (Report). Academy of Medical Sciences. p. 145. ISBN 978-1-903401-18-7.
- Farooq MU, Min J, Goshgarian C, Gorelick PB (September 2017). "Pharmacotherapy for Vascular Cognitive Impairment". CNS Drugs (Review). 31 (9): 759–776. doi:10.1007/s40263-017-0459-3. PMID 28786085. S2CID 23271739.
Other medications have been considered or tried for the treatment of VCI or VaD. These include [...] piracetam. There is no convincing evidence about the efficacy of these medications in the treatment of VCI.
- Malykh AG, Sadaie MR (February 2010). "Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders". Drugs. 70 (3): 287–312. doi:10.2165/11319230-000000000-00000. PMID 20166767. S2CID 12176745.
- Nootropil®. Arzneimittel-Kompendium der Schweiz. 2013-09-12. Retrieved 2013-10-27.
- Al Hajeri A, Fedorowicz Z (February 2016). "Piracetam for reducing the incidence of painful sickle cell disease crises". The Cochrane Database of Systematic Reviews. 2 (4): CD006111. doi:10.1002/14651858.CD006111.pub3. PMC 7390168. PMID 26869149.
- Hofmeyr GJ, Kulier R (June 2012). "Piracetam for fetal distress in labour". The Cochrane Database of Systematic Reviews (6): CD001064. doi:10.1002/14651858.CD001064.pub2. PMC 7048034. PMID 22696322.
- Ricci S, Celani MG, Cantisani TA, Righetti E (September 2012). "Piracetam for acute ischaemic stroke". The Cochrane Database of Systematic Reviews (9): CD000419. doi:10.1002/14651858.CD000419.pub3. PMC 7034527. PMID 22972044.
- Zhang J, Wei R, Chen Z, Luo B (July 2016). "Piracetam for Aphasia in Post-stroke Patients: A Systematic Review and Meta-analysis of Randomized Controlled Trials". CNS Drugs. 30 (7): 575–587. doi:10.1007/s40263-016-0348-1. PMID 27236454. S2CID 22955205.
- Yeo SH, Lim ZI, Mao J, Yau WP (October 2017). "Effects of Central Nervous System Drugs on Recovery After Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials". Clinical Drug Investigation. 37 (10): 901–928. doi:10.1007/s40261-017-0558-4. PMID 28756557. S2CID 6520934.
- Chouinard G, Annable L, Ross-Chouinard A, Olivier M, Fontaine F (1983). "Piracetam in elderly psychiatric patients with mild diffuse cerebral impairment". Psychopharmacology. 81 (2): 100–106. doi:10.1007/BF00429000. PMID 6415738. S2CID 32702769.
- Hakkarainen H, Hakamies L (1978). "Piracetam in the treatment of post-concussional syndrome. A double-blind study". European Neurology. 17 (1): 50–55. doi:10.1159/000114922. PMID 342247.
- Gouliaev AH, Senning A (May 1994). "Piracetam and other structurally related nootropics". Brain Research. Brain Research Reviews. 19 (2): 180–222. doi:10.1016/0165-0173(94)90011-6. PMID 8061686. S2CID 18122566.
- "Piracetam Material Safety Sheet" (PDF). Spectrum.
- Ahmed AH, Oswald RE (March 2010). "Piracetam defines a new binding site for allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors". Journal of Medicinal Chemistry. 53 (5): 2197–2203. doi:10.1021/jm901905j. PMC 2872987. PMID 20163115.
- Giurgea CE (January 1982). "The nootropic concept and its prospective implications". Drug Development Research. 2 (5): 441–446. doi:10.1002/ddr.430020505. ISSN 1098-2299. S2CID 145059666.
- Winnicka K, Tomasiak M, Bielawska A (2005). "Piracetam--an old drug with novel properties?". Acta Poloniae Pharmaceutica. 62 (5): 405–409. PMID 16459490.
- Müller WE, Eckert GP, Eckert A (March 1999). "Piracetam: novelty in a unique mode of action". Pharmacopsychiatry. 32 Suppl 1 (Suppl 1): 2–9. doi:10.1055/s-2007-979230. PMID 10338102.
- Grau M, Montero JL, Balasch J (1987). "Effect of Piracetam on electrocorticogram and local cerebral glucose utilization in the rat". General Pharmacology. 18 (2): 205–211. doi:10.1016/0306-3623(87)90252-7. PMID 3569848.
- Nickolson VJ, Wolthuis OL (October 1976). "Effect of the acquisition-enhancing drug piracetam on rat cerebral energy metabolism. Comparison with naftidrofuryl and methamphetamine". Biochemical Pharmacology. 25 (20): 2241–2244. doi:10.1016/0006-2952(76)90004-6. PMID 985556.
- Tacconi MT, Wurtman RJ (1986). "Piracetam: physiological disposition and mechanism of action". Advances in Neurology. 43: 675–685. PMID 3946121.
- Yeh HH, Yang YH, Ko JY, Chen SH (July 2006). "Rapid determination of piracetam in human plasma and cerebrospinal fluid by micellar electrokinetic chromatography with sample direct injection". Journal of Chromatography A. 1120 (1–2): 27–34. doi:10.1016/j.chroma.2005.11.071. PMID 16343512.
- Bravo-Martínez J, Arenas I, Vivas O, Rebolledo-Antúnez S, Vázquez-García M, Larrazolo A, García DE (October 2012). "A novel CaV2.2 channel inhibition by piracetam in peripheral and central neurons". Experimental Biology and Medicine. 237 (10): 1209–1218. doi:10.1258/ebm.2012.012128. PMID 23045722. S2CID 25909697.
- Li JJ, Corey EJ (2013). Drug Discovery: Practices, Processes, and Perspectives. John Wiley & Sons. p. 276. ISBN 9781118354469.
- Schmidt D, Shorvon S (2016). The End of Epilepsy?: A History of the Modern Era of Epilepsy Research 1860-2010. Oxford University Press. p. 69. ISBN 9780198725909.
- Medew J (1 October 2009). "Call for testing on 'smart drugs'". Fairfax Media. Retrieved 29 May 2014.
- "Erowid Piracetam Vault: Legal Status".
- Bellamy J (26 September 2019). "FDA proposes ban on curcumin and other naturopathic favorites in compounded drugs". Science-Based Medicine.
- http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1547788739542.pdf
- "Nootropil Tablets 800 mg". (emc).
- "UCB's piracetam approved in Japan". The Pharma Letter. 25 November 1999.
- "Guidance Document on the Import Requirements for Health Products under the Food and Drugs Act and its Regulations (GUI-0084)". Health Canada / Health Products and Food Branch Inspectorate. 1 June 2010. Archived from the original on 3 December 2017. Retrieved 15 December 2019.
- UCB Pharma Limited (2005). "Nootropil 800 mg & 1200 mg Tablets and Solution". electronic Medicines Compendium. Datapharm Communications. Archived from the original on 7 December 2006. Retrieved 8 December 2005.
External links
- Gouliaev AH, Senning A (May 1994). "Piracetam and other structurally related nootropics". Brain Research. Brain Research Reviews. 19 (2): 180–222. doi:10.1016/0165-0173(94)90011-6. PMID 8061686. S2CID 18122566.