Median lethal dose
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a toxin, radiation, or pathogen.[1] The value of LD50 for a substance is the dose required to kill half the members of a tested population after a specified test duration. LD50 figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD50 is indicative of increased toxicity.
The test was created by J.W. Trevan in 1927.[2] The term semilethal dose is occasionally used in the same sense, in particular with translations of foreign language text, but can also refer to a sublethal dose. LD50 is usually determined by tests on animals such as laboratory mice. In 2011, the U.S. Food and Drug Administration approved alternative methods to LD50 for testing the cosmetic drug Botox without animal tests.[3][4]
Conventions
The LD50 is usually expressed as the mass of substance administered per unit mass of test subject, typically as milligrams of substance per kilogram of body mass, sometimes also stated as nanograms (suitable for botulinum), micrograms, or grams (suitable for paracetamol) per kilogram. Stating it this way allows the relative toxicity of different substances to be compared, and normalizes for the variation in the size of the animals exposed (although toxicity does not always scale simply with body mass). For substances in the environment, such as poisonous vapors or substances in water that are toxic to fish, the concentration in the environment (per cubic metre or per litre) is used, giving a value of LC50. But in this case, the exposure time is important (see below).
The choice of 50% lethality as a benchmark avoids the potential for ambiguity of making measurements in the extremes and reduces the amount of testing required. However, this also means that LD50 is not the lethal dose for all subjects; some may be killed by much less, while others survive doses far higher than the LD50. Measures such as "LD1" and "LD99" (dosage required to kill 1% or 99%, respectively, of the test population) are occasionally used for specific purposes.[5]
Lethal dosage often varies depending on the method of administration; for instance, many substances are less toxic when administered orally than when intravenously administered. For this reason, LD50 figures are often qualified with the mode of administration, e.g., "LD50 i.v."
The related quantities LD50/30 or LD50/60 are used to refer to a dose that without treatment will be lethal to 50% of the population within (respectively) 30 or 60 days. These measures are used more commonly within radiation health physics, as survival beyond 60 days usually results in recovery.
A comparable measurement is LCt50, which relates to lethal dosage from exposure, where C is concentration and t is time. It is often expressed in terms of mg-min/m3. ICt50 is the dose that will cause incapacitation rather than death. These measures are commonly used to indicate the comparative efficacy of chemical warfare agents, and dosages are typically qualified by rates of breathing (e.g., resting = 10 L/min) for inhalation, or degree of clothing for skin penetration. The concept of Ct was first proposed by Fritz Haber and is sometimes referred to as Haber's law, which assumes that exposure to 1 minute of 100 mg/m3 is equivalent to 10 minutes of 10 mg/m3 (1 × 100 = 100, as does 10 × 10 = 100).
Some chemicals, such as hydrogen cyanide, are rapidly detoxified by the human body, and do not follow Haber's law. So, in these cases, the lethal concentration may be given simply as LC50 and qualified by a duration of exposure (e.g., 10 minutes). The Material Safety Data Sheets for toxic substances frequently use this form of the term even if the substance does follow Haber's law.
For disease-causing organisms, there is also a measure known as the median infective dose and dosage. The median infective dose (ID50) is the number of organisms received by a person or test animal qualified by the route of administration (e.g., 1,200 org/man per oral). Because of the difficulties in counting actual organisms in a dose, infective doses may be expressed in terms of biological assay, such as the number of LD50's to some test animal. In biological warfare infective dosage is the number of infective doses per cubic metre of air times the number of minutes of exposure (e.g., ICt50 is 100 medium doses - min/m3).
Limitation
As a measure of toxicity, LD50 is somewhat unreliable and results may vary greatly between testing facilities due to factors such as the genetic characteristics of the sample population, animal species tested, environmental factors and mode of administration.[6]
There can be wide variability between species as well; what is relatively safe for rats may very well be extremely toxic for humans (cf. paracetamol toxicity), and vice versa. For example, chocolate, comparatively harmless to humans, is known to be toxic to many animals. When used to test venom from venomous creatures, such as snakes, LD50 results may be misleading due to the physiological differences between mice, rats, and humans. Many venomous snakes are specialized predators on mice, and their venom may be adapted specifically to incapacitate mice; and mongooses may be exceptionally resistant. While most mammals have a very similar physiology, LD50 results may or may not have equal bearing upon every mammal species, such as humans, etc.
Examples
Note: Comparing substances (especially drugs) to each other by LD50 can be misleading in many cases due (in part) to differences in effective dose (ED50). Therefore, it is more useful to compare such substances by therapeutic index, which is simply the ratio of LD50 to ED50.
The following examples are listed in reference to LD50 values, in descending order, and accompanied by LC50 values, {bracketed}, when appropriate.
| Substance | Animal, route | LD50 {LC50} |
LD50 : g/kg {LC50 : g/L} standardised |
Reference |
|---|---|---|---|---|
| Water (H2O) | rat, oral | >90,000 mg/kg | >90 | [7] |
| Sucrose (table sugar) | rat, oral | 29,700 mg/kg | 29.7 | [8] |
| Corn Syrup | rat, oral | 25,800 mg/kg | 25.8 | [9] |
| Glucose (blood sugar) | rat, oral | 25,800 mg/kg | 25.8 | [10] |
| Monosodium glutamate (MSG) | rat, oral | 16,600 mg/kg | 16.6 | [11] |
| Stevioside (from stevia) | mice and rats, oral | 15,000 mg/kg | 15 | [12] |
| Gasoline (petrol) | rat | 14,063 mg/kg | 14.0 | [13] |
| Vitamin C (ascorbic acid) | rat, oral | 11,900 mg/kg | 11.9 | [14] |
| Glyphosate (isopropylamine salt of) | rat, oral | 10,537 mg/kg | 10.537 | [15] |
| Lactose (milk sugar) | rat, oral | 10,000 mg/kg | 10 | [16] |
| Aspartame | mice, oral | 10,000 mg/kg | 10 | [17] |
| Urea (OC(NH2)2) | rat, oral | 8,471 mg/kg | 8.471 | [18] |
| Cyanuric acid | rat, oral | 7,700 mg/kg | 7.7 | [19] |
| Cadmium sulfide (CdS) | rat, oral | 7,080 mg/kg | 7.08 | [20] |
| Ethanol (CH3CH2OH) | rat, oral | 7,060 mg/kg | 7.06 | [21] |
| Sodium isopropyl methylphosphonic acid (IMPA, metabolite of sarin) | rat, oral | 6,860 mg/kg | 6.86 | [22] |
| Melamine | rat, oral | 6,000 mg/kg | 6 | [19] |
| Taurine | rat, oral | 5,000 mg/kg | 5 | [23] |
| Melamine cyanurate | rat, oral | 4,100 mg/kg | 4.1 | [19] |
| Fructose (fruit sugar) | rat, oral | 4,000 mg/kg | 4 | [24] |
| Sodium molybdate (Na2MoO4) | rat, oral | 4,000 mg/kg | 4 | [25] |
| Sodium chloride (table salt) | rat, oral | 3,000 mg/kg | 3 | [26] |
| Paracetamol (acetaminophen) | rat, oral | 1,944 mg/kg | 1.944 | [27] |
| Delta-9-tetrahydrocannabinol (THC) | rat, oral | 1,270 mg/kg | 1.27 | [28] |
| Cannabidiol (CBD) | rat, oral | 980 mg/kg | 0.98 | [29] |
| Methanol (CH3OH) | human, oral | 810 mg/kg | 0.81 | [30] |
| Arsenic (As) | rat, oral | 763 mg/kg | 0.763 | [31] |
| Ibuprofen | rat, oral | 636 mg/kg | 0.636 | [32] |
| Formaldehyde (CH2O) | rat, oral | 600–800 mg/kg | 0.6 | [33] |
| Solanine (main alkaloid in the several plants in Solanaceae amongst them Solanum tuberosum) | rat, oral (2.8 mg/kg human, oral) | 590 mg/kg | 0.590 | [34] |
| Alkyl dimethyl benzalkonium chloride (ADBAC) | rat, oral fish, immersion aquatic invertebrates, immersion |
304.5 mg/kg {0.28 mg/L} {0.059 mg/L} |
0.3045 {0.00028} {0.000059} |
[35] |
| Coumarin (benzopyrone, from Cinnamomum aromaticum and other plants) | rat, oral | 293 mg/kg | 0.293 | [36] |
| Psilocybin (from magic mushrooms) | mouse, oral | 280 mg/kg | 0.280 | [37] |
| Hydrochloric acid (HCl) | rat, oral | 238–277 mg/kg | 0.238 | [38] |
| Ketamine | rat, intraperitoneal | 229 mg/kg | 0.229 | [39] |
| Aspirin (acetylsalicylic acid) | rat, oral | 200 mg/kg | 0.2 | [40] |
| Caffeine | rat, oral | 192 mg/kg | 0.192 | [41] |
| Arsenic trisulfide (AsS3) | rat, oral | 185–6,400 mg/kg | 0.185–6.4 | [42] |
| Sodium nitrite (NaNO2) | rat, oral | 180 mg/kg | 0.18 | [43] |
| Methylenedioxymethamphetamine (MDMA, ecstasy) | rat, oral | 160 mg/kg | 0.18 | [44] |
| Uranyl acetate dihydrate (UO2(CH3COO)2) | mouse, oral | 136 mg/kg | 0.136 | [45] |
| Dichlorodiphenyltrichloroethane (DDT) | mouse, oral | 135 mg/kg | 0.135 | [46] |
| Uranium (U) | mice, oral | 114 mg/kg (estimated) | 0.114 | [45] |
| Bisoprolol | mouse, oral | 100 mg/kg | 0.1 | [47] |
| Cocaine | mouse, oral | 96 mg/kg | 0.096 | [48] |
| Cobalt(II) chloride (CoCl2) | rat, oral | 80 mg/kg | 0.08 | [49] |
| Cadmium oxide (CdO) | rat, oral | 72 mg/kg | 0.072 | [50] |
| Thiopental sodium (used in lethal injection) | rat, oral | 64 mg/kg | 0.064 | [51] |
| Demeton-S-methyl | rat, oral | 60 mg/kg | 0.060 | [52] |
| Methamphetamine | rat, intraperitoneal | 57 mg/kg | 0.057 | [53] |
| Sodium fluoride (NaF) | rat, oral | 52 mg/kg | 0.052 | [54] |
| Nicotine | mouse and rat, oral
human, smoking |
50 mg/kg | 0.05 | [55] |
| Pentaborane | human, oral | 50 mg/kg | 0.05 | [56] |
| Capsaicin | mouse, oral | 47.2 mg/kg | 0.0472 | [57] |
| Vitamin D3 (cholecalciferol) | rat, oral | 37 mg/kg | 0.037 | [58] |
| Piperidine (from black pepper) | rat, oral | 30 mg/kg | 0.030 | [59] |
| Heroin (diamorphine) | mouse, intravenous | 21.8 mg/kg | 0.0218 | [60] |
| Lysergic acid diethylamide (LSD) | rat, intravenous | 16.5 mg/kg | 0.0165 | [61] |
| Arsenic trioxide (As2O3) | rat, oral | 14 mg/kg | 0.014 | [62] |
| Metallic arsenic (As) | rat, intraperitoneal | 13 mg/kg | 0.013 | [63] |
| Sodium cyanide (NaCN) | rat, oral | 6.4 mg/kg | 0.0064 | [64] |
| Chlorotoxin (CTX, from scorpions) | mice | 4.3 mg/kg | 0.0043 | [65] |
| Hydrogen cyanide (HCN) | mouse, oral | 3.7 mg/kg | 0.0037 | [66] |
| Carfentanil | rat, intravenous | 3.39 mg/kg | 0.00339 | [67] |
| Nicotine (from various Solanaceae genera) | mice, oral | 3.3 mg/kg | 0.0033 | [55] |
| White phosphorus (P) | rat, oral | 3.03 mg/kg | 0.00303 | [68] |
| Strychnine (from Strychnos nux-vomica) | human, oral | 1–2 mg/kg (estimated) | 0.001–0.002 | [69] |
| Mercury(II) chloride (HgCl2) | rat, oral | 1 mg/kg | 0.001 | [70] |
| Nicotine | human, oral | 0.8 mg/kg (estimated) | 0.0008 | [55] |
| Cantharidin (from blister beetles) | human, oral | 500 μg/kg | 0.0005 | [71] |
| Aflatoxin B1 (from Aspergillus flavus mold) | rat, oral | 480 μg/kg | 0.00048 | [72] |
| Plutonium (Pu) | dog, intravenous | 320 μg/kg | 0.00032 | [73] |
| Amatoxin (from Amanita phalloides mushrooms) | rat | 300-700 μg/kg | 0.0007 | [74] |
| Bufotoxin (from Bufo toads) | cat, intravenous | 300 μg/kg | 0.0003 | [75] |
| Caesium-137 (137 Cs) |
mouse, parenteral | 21.5 μCi/g | 0.000245 | [76] |
| Sodium fluoroacetate (CH2FCOONa) | rat, oral | 220 μg/kg | 0.00022 | [77] |
| Chlorine trifluoride (ClF3) | mouse, absorption through skin | 178 μg/kg | 0.000178 | [78] |
| Sarin | mouse, subcutaneous injection | 172 μg/kg | 0.000172 | [79] |
| Robustoxin (from Sydney funnel-web spider) | mice | 150 μg/kg | 0.000150 | [80] |
| VX | human, oral, inhalation, absorption through skin/eyes | 140 μg/kg (estimated) | 0.00014 | [81] |
| Venom of the Brazilian wandering spider | rat, subcutaneous | 134 μg/kg | 0.000134 | [82] |
| Aconitine (from Aconitum napellus and related species) | rat, intraveneous | 80 μg/kg | 0.000080 | [83] |
| Dimethylmercury (Hg(CH3)2) | human, transdermal | 50 μg/kg | 0.000050 | [84] |
| TBPO (t-Butyl-bicyclophosphate) | mouse, intravenous | 36 μg/kg | 0.000036 | [85] |
| Fentanyl | monkey | 30 μg/kg | 0.00003 | [86] |
| Venom of the Inland Taipan (Australian snake) | rat, subcutaneous | 25 μg/kg | 0.000025 | [87] |
| Ricin (from castor oil plant) | rat, intraperitoneal rat, oral |
22 μg/kg 20–30 mg/kg |
0.000022 0.02 |
[88] |
| 2,3,7,8-Tetrachlorodibenzodioxin (TCDD, in Agent Orange) | rat, oral | 20 μg/kg | 0.00002 | |
| Tetrodotoxin from the blue-ringed octopus | intravenous | 8.2 μg/kg | 0.0000082 | [89] |
| CrTX-A (from Carybdea rastonii box jellyfish venom) | crayfish, intraperitoneal | 5 μg/kg | 0.000005 | [90] |
| Latrotoxin (from widow spider venom) | mice | 4.3 μg/kg | 0.0000043 | [91] |
| Epibatidine (from Epipedobates anthonyi poison dart frog) | mouse, intravenous | 1.46-13.98 μg/kg | 0.00000146 | [92] |
| Batrachotoxin (from poison dart frog) | human, sub-cutaneous injection | 2–7 μg/kg (estimated) | 0.000002 | [93] |
| Abrin (from rosary pea) | mice, intravenously
human, inhalation human, oral |
0.7 μg/kg
3.3 μg/kg 10–1000 μg/kg |
0.0000007
0.0000033 0.00001–0.001 | |
| Saxitoxin (from certain marine dinoflagellates) | human, intravenously
human, oral |
0.6 μg/kg
5.7 μg/kg |
0.0000006
0.0000057 |
[93] |
| Pacific Ciguatoxin-1 (from ciguateric fish) | mice, intraperitoneal | 250 ng/kg | 0.00000025 | [94] |
| Palytoxin (from Palythoa coral) | mouse, intravenous | 45 ng/kg
2.3–31.5 μg/kg |
0.000000045
0.0000023 |
[95] |
| Maitotoxin (from ciguateric fish) | mouse, intraperitoneal | 50 ng/kg | 0.00000005 | [96] |
| Polonium-210 (210 Po) |
human, inhalation | 10 ng/kg (estimated) | 0.00000001 | [97] |
| Diphtheria toxin (from Corynebacterium) | mice | 10 ng/kg | 0.00000001 | [98] |
| Shiga toxin (from Shigella bacteria) | mice | 2 ng/kg | 0.000000002 | [98] |
| Tetanospasmin (from Clostridium tetani) | mice | 2 ng/kg | 0.000000002 | [98] |
| Botulinum toxin (from Clostridium botulinum) | human, oral, injection, inhalation | 1 ng/kg (estimated) | 0.000000001 | [99] |
| Ionizing radiation | human, irradiation | 5 Gy (Gray) | — | [100] |
Poison scale
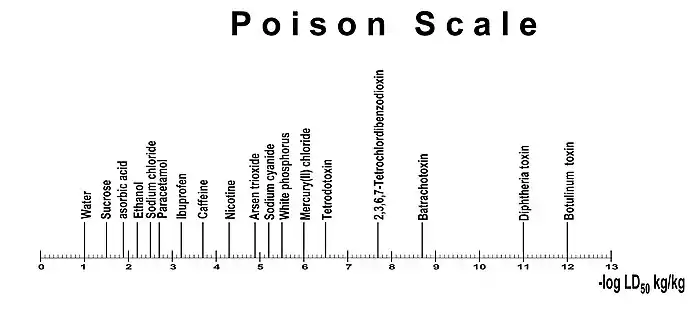
The LD50 values have a very wide range. The botulinum toxin as the most toxic substance known has an LD50 value of 1 ng/kg, while the most non-toxic substance water has an LD50 value of more than 90 g/kg. That's a difference of about 1 in 100 billion or 11 orders of magnitude. As with all measured values that differ by many orders of magnitude, a logarithmic view is advisable. Well-known examples are the indication of the earthquake strength using the Richter scale, the pH value, as a measure for the acidic or basic character of an aqueous solution or of loudness in decibels . In this case, the negative decimal logarithm of the LD50 values, which is standardized in kg per kg body weight, is considered −log10(LD50).
The dimensionless value found can be entered in a toxin scale. Water as the baseline substance is neatly 1 in the negative logarithmic toxin scale.
Animal rights concerns
Animal-rights and animal-welfare groups, such as Animal Rights International,[102] have campaigned against LD50 testing on animals. Several countries, including the UK, have taken steps to ban the oral LD50, and the Organisation for Economic Co-operation and Development (OECD) abolished the requirement for the oral test in 2001 (see Test Guideline 401, Trends in Pharmacological Sciences Vol 22, February 22, 2001).
See also
- Animal testing
- Reed-Muench method
- The dose makes the poison – the toxicology adage that high quantities of any substance is lethal
Other measures of toxicity
- IDLH
- Certain safety factor
- Therapeutic index
- Protective index
- Fixed Dose Procedure to estimate LD50
- Median toxic dose (TD50)
- Lowest published lethal dose (LDLo)
- EC50 (half maximal effective concentration)
- IC50 (half maximal inhibitory concentration)
- Draize test
- Indicative limit value
- No-observed-adverse-effect level (NOAEL)
- Lowest-observed-adverse-effect level (LOAEL)
- Up-and-down procedure
Related measures
- TCID50 Tissue Culture Infective Dosage
- Plaque forming units (pfu)
References
- "Absolute lethal dose (LD100)". IUPAC Gold Book. International Union of Pure and Applied Chemistry. Archived from the original on 2019-07-01. Retrieved 2019-07-01.
- "What is a LD50 and LC50?". OSH Answers Fact Sheets. Canadian Centre for Occupational Health and Safety. 5 October 2021.
- "Allergan Receives FDA Approval for First-of-Its-Kind, Fully in vitro, Cell-Based Assay for BOTOX and BOTOX Cosmetic (onabotulinumtoxinA)". Allergan Web site. 24 June 2011. Archived from the original on 26 June 2011. Retrieved 2012-08-15.
- Gaul GM (12 April 2008). "In U.S., Few Alternatives To Testing On Animals". Washington Post. Retrieved 2011-06-26.
- Doris V. Sweet, ed. (July 1997). "Registry of Toxic Effects of Chemical Substances (RTECS) / Comprehensive Guide to the RTECS" (PDF). U.S. Department of Health and Human Services. DHHS (NIOSH) Publication No. 97-119. Archived from the original (PDF) on 2013-05-16.
- Ernest Hodgson (2004). A Textbook of Modern Toxicology. Wiley-Interscience (3rd ed.).
- "Material Safety Data Sheet Water MSDS". Section 11: Toxicological Information for the LD50 verification. Archived from the original on 2012-09-02. Retrieved 2012-05-09.
- "Safety (MSDS) data for sucrose". ox.ac.uk. Archived from the original on 2011-06-12.
- "Safety (MSDS) data for Corn Syrup". fishersci.com. Retrieved 2022-09-21.
- "Safety (MSDS) data for glucose" (PDF). utoronto.ca. Archived from the original (PDF) on 2017-01-01. Retrieved 2016-12-31.
- Walker R, Lupien JR (April 2000). "The safety evaluation of monosodium glutamate". The Journal of Nutrition. 130 (4S Suppl): 1049S–52S. doi:10.1093/jn/130.4.1049S. PMID 10736380.
- Toskulkao C, Chaturat L, Temcharoen P, Glinsukon T (1997). "Acute toxicity of stevioside, a natural sweetener, and its metabolite, steviol, in several animal species". Drug and Chemical Toxicology. 20 (1–2): 31–44. doi:10.3109/01480549709011077. PMID 9183561.
- "Toxicological profile for gasoline" (PDF). U.S. Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry. June 1995. p. 47. Archived from the original (PDF) on 2017-05-15. Retrieved 2020-01-05.
- "Safety (MSDS) data for ascorbic acid". Oxford University. 2005-10-09. Archived from the original on 2007-02-09. Retrieved 2007-02-21.
- "Glyphosate-isopropylammonium". PubChem.
- "Safety (MSDS) data for Lactose" (PDF). Archived from the original (PDF) on 2016-08-03. Retrieved 2016-12-31.
- "Material Safety Data Sheet: Aspartame" (PDF). Spectrum. Archived from the original (PDF) on 2016-12-26.
- "Safety (MSDS) data for urea". 2015-03-06. Section 11: Toxicological Information for the LD50 verification. Archived from the original on 2015-03-01. Retrieved 2015-03-06.
- A.A. Babayan, A.V.Aleksandryan, "Toxicological characteristics of melamine cyanurate, melamine and cyanuric acid", Zhurnal Eksperimental'noi i Klinicheskoi Meditsiny, Vol.25, 345–9 (1985). Original article in Russian.
- Advanced Search – Alfa Aesar – A Johnson Matthey Company Archived 2015-07-24 at the Wayback Machine. Alfa.com. Retrieved on 2013-07-17.
- "Safety (MSDS) data for ethyl alcohol". ox.ac.uk. Archived from the original on 2011-07-14.
- Mecler FJ (May 1981). Mammalian Toxological Evaluation of DIMP and DCBP (Phase 3 – IMPA) (Final report). Litton Bionetics, Inc. Archived from the original on October 4, 2013.
The oral LD50 values for the test material, IMPA, were 7650 and 6070 mg/kg for male and female rats, respectively.
- "Safety data for taurine" (PDF). scbt.com. Archived from the original (PDF) on 2017-01-18. Retrieved 2017-01-18.
- "Safety (MSDS) data for fructose". sciencelab.com. Archived from the original on 2017-07-02. Retrieved 2016-12-31.
- "Safety (MSDS) data for sodium molybdate". ox.ac.uk. Archived from the original on 2011-01-28.
- "Safety (MSDS) data for sodium chloride". ox.ac.uk. Archived from the original on 2011-06-07.
- "Safety (MSDS) data for paracetamol". Millipore Sigma. Merck KGaA.
- Rosenkrantz H, Heyman IA, Braude MC (April 1974). "Inhalation, parenteral and oral LD50 values of Δ9-tetrahydrocannabinol in Fischer rats". Toxicology and Applied Pharmacology. 28 (1): 18–27. doi:10.1016/0041-008X(74)90126-4. PMID 4852457.
- "MSDS of CBD" (PDF). chemblink.com. Archived from the original (PDF) on 2016-12-26. Retrieved 2016-12-26.
- "Methanol Poisoning Overview". antizol.com. Archived from the original on 2011-10-05.
- "Arsenic". PubChem.
- "Ibuprofen – National Library of Medicine HSDB Database". toxnet.nlm.nih.gov.
- "Formaldehyde SIDS Initial Assessment Report" (PDF). inchem.org. Archived from the original (PDF) on 2018-06-13. Retrieved 2016-12-26.
- "Solanine – National Library of Medicine HSDB Database". toxnet.nlm.nih.gov.
- Frank T. Sanders, ed. (August 2006). Reregistration Eligibility Decision for Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC) (PDF) (Report). U.S. Environmental Protection Agency Office of Prevention, Pesticides, and Toxic Substances. p. 114. Archived from the original (PDF) on 2009-10-24. Retrieved 2009-03-31.
- Coumarin Material Safety Data Sheet (MSDS) Archived 2004-10-21 at the Wayback Machine
- Rumack BH, Spoerke DJ (27 September 1994). Handbook of Mushroom Poisoning: Diagnosis and Treatment. CRC Press. ISBN 978-0-8493-0194-0 – via Google Books.
- "Material Safety Data Sheet: Hydrochloric acid 32-38% solution". Fisher. 1 April 2008.
- "Ketamine" (PDF). nih.gov.
- "Safety (MSDS) data for acetylsalicylic acid". ox.ac.uk. Archived from the original on 2011-07-16.
- Boyd EM (May 1959). "The acute oral toxicity of caffeine". Toxicology and Applied Pharmacology. 1 (3): 250–7. doi:10.1016/0041-008X(59)90109-7. PMID 13659532.
- "Material Safety Data Sheet – Spent Metal Catalyst" (PDF). Archived from the original (PDF) on 2011-09-28.
- "Safety (MSDS) data for sodium nitrite". ox.ac.uk.
- Gable RS (September 2004). "Acute toxic effects of club drugs". Journal of Psychoactive Drugs. 36 (3): 303–13. doi:10.1080/02791072.2004.10400031. PMID 15559678. S2CID 30689421.
- "Chemical toxicity of uranium" (PDF). who.int.
- Hayes WJ, Simmons SW, Knipling EF (1959). "Dose-Mortality Relationships in Animals". DDT: The Insecticide Dichlorodiphenyltrichloroethane and Its Significance / Das Insektizid Dichlordiphenyltrichloräthan und Seine Bedeutung. pp. 18–40. doi:10.1007/978-3-0348-6809-9_3. ISBN 978-3-0348-6796-2.
- "Bisoprolol". www.drugbank.ca.
- "Cocaine". www.drugbank.ca. Archived from the original on 2016-11-20. Retrieved 2016-12-26.
- "Safety (MSDS) data for cobalt (II) chloride". ox.ac.uk. Archived from the original on 2011-04-07.
- Safety (MSDS) data for cadmium oxide
- "Thiopental sodium". Pubchem.
- "Demeton-s-methyl". Extoxnet. September 1995.
- Kiyatkin EA, Sharma HS (2009). "Acute Methamphetamine Intoxication". New Concepts of Psychostimulant Induced Neurotoxicity. International Review of Neurobiology. Vol. 88. pp. 65–100. doi:10.1016/S0074-7742(09)88004-5. ISBN 978-0-12-374504-0. PMC 3145326. PMID 19897075.
- "Sodium fluoride". hazard.com. Archived from the original on 2011-09-28. Retrieved 2011-07-31.
- Mayer B (January 2014). "How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century". Archives of Toxicology. 88 (1): 5–7. doi:10.1007/s00204-013-1127-0. PMC 3880486. PMID 24091634.
- "Pentaborane chemical and safety data" (PDF). noaa.gov.
- "Capsaicin Material Safety Data Sheet". sciencelab.com. 2007. Archived from the original (PDF) on 2007-09-29. Retrieved 2007-07-13.
- "MSDS for cholecalciferol crystalline" (PDF). hmdb.ca. Archived from the original (PDF) on 2016-12-26. Retrieved 2016-12-26.
- "Material Safety Data Sheet: Piperidine". Fisher. 29 October 2007.
- "Diamorphine (PIM 261F, French)". www.inchem.org. Archived from the original on 2016-05-02. Retrieved 2016-12-26.
- Erowid LSD (Acid) Vault : Fatalities / Deaths. Erowid.org. Retrieved on 2013-07-17.
- "Safety (MSDS) data for arsenic trioxide". ox.ac.uk. Archived from the original on 2010-03-09.
- "Safety (MSDS) data for metallic arsenic". ox.ac.uk. Archived from the original on 2011-01-14.
- "Safety (MSDS) data for sodium cyanide". ox.ac.uk. Archived from the original on 2009-01-13.
- "Chlorotoxin: A Helpful Natural Scorpion Peptide to Diagnose Glioma and Fight Tumor Invasion".
- "Safety (MSDS) data for hydrogen cyanide" (PDF). orica.com. Archived from the original (PDF) on 2016-12-26. Retrieved 2016-12-26.
- "Critical Review Carfentanil" (PDF). Retrieved 2019-01-31.
- "Hexachloroethane" (PDF). Retrieved 2014-01-03.
- INCHEM: Chemical Safety Information from Intergovernmental Organizations: Strychnine.
- "Mercuric Chloride Safety Data Sheet" (PDF). LabChem. p. 6. Archived from the original (PDF) on 2019-11-26. Retrieved 2020-01-06.
- Meister RT, Sine C (2013). Crop Protection Handbook. Vol. 99. Willoughby, Ohio: Meister Pub Co. p. 664. ISBN 978-1892829269.
- "Safety (MSDS) data for aflatoxin B1". ox.ac.uk. Archived from the original on 2010-08-11.
- Voelz GL, Buican IG (2000). "Plutonium and Health — How great is the risk?" (PDF). Los Alamos Science (26): 74–89.
- Wong JH, Ng TB (2006). "Toxins from Basidiomycete Fungi (Mushroom): Amatoxins, Phallotoxins, and Virotoxins". In Kastin AJ (ed.). Handbook of Biologically Active Peptides. pp. 131–135. doi:10.1016/B978-012369442-3/50023-4. ISBN 978-0-12-369442-3.
- "Bufotoxin". ChemIDplus. U.S. National Library of Medicine.
- Moskalev YI (1961). "Biological Effects of Cesium-137". In Lebedinskiĭ AV, Moskalev YI (eds.). Distribution, Biological Effects, and Migration of Radioactive Isotopes. Translation Series. United States Atomic Energy Commission (published April 1974). p. 220. AEC-tr-7512. [(21.5 μCi/g) × (1000 g/kg) × (0.0114 μg/μCi) = 245 μg/kg]
- Meister R, Since C (2013). Crop Protection Handbook 2013. Willoughby, Ohio: Meister Pub Co. p. 664. ISBN 9781892829269.
- "CDC - Immediately Dangerous to Life or Health Concentrations (IDLH): Chlorine trifluoride - NIOSH Publications and Products". www.cdc.gov. 2018-11-02. Retrieved 2022-07-13.
- Inns RH, Tuckwell NJ, Bright JE, Marrs TC (July 1990). "Histochemical demonstration of calcium accumulation in muscle fibres after experimental organophosphate poisoning". Human & Experimental Toxicology. 9 (4): 245–50. doi:10.1177/096032719000900407. PMID 2390321. S2CID 20713579.
- Sheumack DD, Baldo BA, Carroll PR, Hampson F, Howden ME, Skorulis A (1984). "A comparative study of properties and toxic constituents of funnel web spider (Atrax) venoms". Comparative Biochemistry and Physiology. C, Comparative Pharmacology and Toxicology. 78 (1): 55–68. doi:10.1016/0742-8413(84)90048-3. PMID 6146485.
- Munro N (January 1994). "Toxicity of the organophosphate chemical warfare agents GA, GB, and VX: implications for public protection". Environmental Health Perspectives. 102 (1): 18–38. doi:10.1289/ehp.9410218. PMC 1567233. PMID 9719666.
- Venomous Animals and their Venoms, vol. III, ed. Wolfgang Bücherl and Eleanor Buckley
- "Aconitine – National Library of Medicine HSDB Database". toxnet.nlm.nih.gov.
- Blayney MB (February 2001). "The need for empirically derived permeation data for personal protective equipment: the death of Dr. Karen E. Wetterhahn". Applied Occupational and Environmental Hygiene. 16 (2): 233–6. doi:10.1080/104732201460389. PMID 11217716.
- Milbrath DS, Engel JL, Verkade JG, Casida JE (February 1979). "Structure--toxicity relationships of 1-substituted-4-alkyl-2,6,7-trioxabicyclo[2.2.2.]octanes". Toxicology and Applied Pharmacology. 47 (2): 287–93. doi:10.1016/0041-008x(79)90323-5. PMID 452023.
- "Fentanyl". www.drugbank.ca. Archived from the original on 2017-07-11. Retrieved 2017-09-29.
- LD50 for various snakes Archived 2012-02-01 at the Wayback Machine. Seanthomas.net. Retrieved on 2013-07-17.
- "Ricin (from Ricinus communis) as undesirable substances in animal feed - Scientific Opinion of the Panel on Contaminants in the Food Chain". EFSA Journal. 6 (9): 726. 2008. CiteSeerX 10.1.1.333.8413. doi:10.2903/j.efsa.2008.726.
- Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, et al. (April 2017). "Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods". EFSA Journal. 15 (4): e04752. doi:10.2903/j.efsa.2017.4752. PMC 7010203. PMID 32625458. S2CID 54043321.
- Nagai H (2003). "Recent Progress in Jellyfish Toxin Study". Journal of Health Science. 49 (5): 337–340. doi:10.1248/jhs.49.337.
- Henderson N, Wright K, Morgan D, Tantum P. "Black Widow Venom (α-Latrotoxin)". Archived from the original (pptx) on 2016-12-26. Retrieved 2016-12-26.
- Sihver W, Långström B, Nordberg A (2000). "Ligands for in vivo imaging of nicotinic receptor subtypes in Alzheimer brain". Acta Neurologica Scandinavica. Supplementum. 176 (s176): 27–33. doi:10.1034/j.1600-0404.2000.00304.x. PMID 11261802. S2CID 23541883.
- Patocka J, Streda L (2002). "Brief review of natural nonprotein neurotoxins". ASA Newsletter. 2 (2): 16–24.
- Caillaud A, de la Iglesia P, Darius HT, Pauillac S, Aligizaki K, Fraga S, et al. (June 2010). "Update on methodologies available for ciguatoxin determination: perspectives to confront the onset of ciguatera fish poisoning in Europe". Marine Drugs. 8 (6): 1838–907. doi:10.3390/md8061838. PMC 2901828. PMID 20631873.
- Ramos V, Vasconcelos V (June 2010). "Palytoxin and analogs: biological and ecological effects". Marine Drugs. 8 (7): 2021–37. doi:10.3390/md8072021. PMC 2920541. PMID 20714422.
- "PubChem Compound Summary for CID 71460273, Maitotoxin". PubChem. National Center for Biotechnology Information.
- Topic 2 Toxic Chemicals and Toxic Effects Archived 2007-09-29 at the Wayback Machine
- Toolson E. "Representative LD50 Values" (PDF). Archived from the original (PDF) on 2015-04-12. Retrieved 2016-12-26.
- Fleming DO, Hunt DL (2000). Biological Safety: principles and practices. Washington, DC: ASM Press. p. 267. ISBN 978-1-55581-180-8.
- Winfried K (2013). "Lethal dose". www.euronuclear.org. Archived from the original on 2018-08-04. Retrieved 2018-09-15.
- Strey, Karsten (December 2019). "Die Gifte-Skala". Chemie in unserer Zeit. 53 (6): 386–399. doi:10.1002/ciuz.201900828. S2CID 199067092.
- Thirty-Two Years of Measurable Change Archived 2007-02-11 at the Wayback Machine
External links
- Canadian Centre for Occupational Health and Safety
- Lipnick RL, Cotruvo JA, Hill RN, Bruce RD, Stitzel KA, Walker AP, et al. (March 1995). "Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures". Food and Chemical Toxicology. 33 (3): 223–31. doi:10.1016/0278-6915(94)00136-C. PMID 7896233.