Sodium nitrite
 | |||
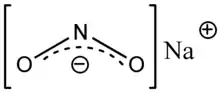 | |||
| |||
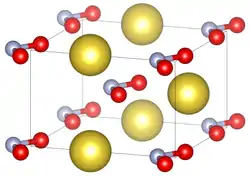 Unit cell of sodium nitrite under standard conditions. | |||
| Identifiers | |||
|---|---|---|---|
CAS Number |
|||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.687 | ||
| EC Number |
| ||
| E number | E250 (preservatives) | ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1500 3287 | ||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
NaNO2 | ||
| Molar mass | 68.9953 g/mol | ||
| Appearance | white or slightly yellowish solid | ||
| Density | 2.168 g/cm3 | ||
| Melting point | 271 °C (520 °F; 544 K) (decomposes at 320 °C) | ||
Solubility in water |
71.4 g/100 mL (0 °C) 84.8 g/100 mL (25 °C) 160 g/100 mL (100 °C) | ||
| Solubility | soluble in methanol (4.4 g/100 mL) ethanol slightly soluble in diethyl ether (0.3 g/100 mL) very soluble in ammonia | ||
| Acidity (pKa) | ~9 | ||
Magnetic susceptibility (χ) |
−14.5·10−6 cm3/mol | ||
Refractive index (nD) |
1.65 | ||
| Structure[1] | |||
Crystal structure |
orthorhombic | ||
Space group |
Im2m | ||
Lattice constant |
a = 3.5653(8) Å, b = 5.5728(7) Å, c = 5.3846(13) Å | ||
Formula units (Z) |
2 | ||
| Thermochemistry | |||
Std molar entropy (S⦵298) |
106 J/mol K | ||
Std enthalpy of formation (ΔfH⦵298) |
−359 kJ/mol[2] | ||
Gibbs free energy (ΔfG⦵) |
−295 kJ/mol | ||
| Pharmacology | |||
| V03AB08 (WHO) | |||
| Hazards | |||
| GHS labelling:[3] | |||
Pictograms |
   | ||
Signal word |
Danger | ||
Hazard statements |
H272, H301, H319, H400 | ||
Precautionary statements |
P220, P273, P301+P310, P305+P351+P338 | ||
| NFPA 704 (fire diamond) | |||
Autoignition temperature |
489 °C (912 °F; 762 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
180 mg/kg (rats, oral) | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Other anions |
Sodium nitrate | ||
Other cations |
Potassium nitrite Ammonium nitrite Lithium nitrite | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Sodium nitrite is an inorganic compound with the chemical formula NaNO2. It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite salt. It is a precursor to a variety of organic compounds, such as pharmaceuticals, dyes, and pesticides, but it is probably best known as a food additive used in processed meats and (in some countries) in fish products.[4]
Uses
Industrial chemistry
The main use of sodium nitrite is for the industrial production of organonitrogen compounds. It is a reagent for conversion of amines into diazo compounds, which are key precursors to many dyes, such as diazo dyes. Nitroso compounds are produced from nitrites. These are used in the rubber industry.[4]
It is used in a variety of metallurgical applications, for phosphatizing and detinning.[4]
Sodium nitrite is an effective corrosion inhibitor and is used as an additive in industrial greases,[5] as an aqueous solution in closed loop cooling systems, and in a molten state as a heat transfer medium.[6]
Food additive and preservative
Sodium nitrite is used to speed up the curing of meat and also impart an attractive pink color.[7][8] Nitrite reacts with the meat myoglobin to cause color changes, first converting to nitrosomyoglobin (bright red), then, on heating, to nitrosohemochrome (a pink pigment).[9]
Historically, salt has been used for the preservation of meat. The salt-preserved meatproduct was usually brownish-gray in color. When sodium nitrite is added with the salt, the meat develops a red, then pink color, which is associated with cured meats such as ham, bacon, hot dogs, and bologna.[10]
In the early 1900s, irregular curing was commonplace. This led to further research surrounding the use of sodium nitrite as an additive in food, standardizing the amount present in foods to minimize the amount needed while maximizing its food additive role.[11] Through this research, sodium nitrite has been found to give taste and color to the meat and inhibit lipid oxidation that leads to rancidity, with varying degrees of effectiveness for controlling growth of disease-causing microorganisms.[11] The ability of sodium nitrite to address the above-mentioned issues has led to production of meat with extended storage life and has improved desirable color and taste. According to scientists working for the meat industry,[12] nitrite has improved food safety.[11] This view is disputed in the light of its ineffectiveness against botulism and the possible carcinogenic effects caused by adding nitrites to meat.[7]
Nitrite has the E number E250. Potassium nitrite (E249) is used in the same way. It is approved for usage in the EU,[13][14] USA[15] and Australia and New Zealand.[16]
In meat-processing, sodium nitrite is never used in a pure state but always mixed with common salt. This mixture is known as nitrited salt, curing salt or nitrited curing salt. In Europe, nitrited curing salt contains between 99.1% and 99.5% common salt and between 0.5% and 0.9% nitrite. In the US, nitrited curing salt is dosed at 6% and must be remixed with salt before use.[17]
Color and taste
The appearance and taste of meat is an important component of consumer acceptance.[11] Sodium nitrite is responsible for the desirable red color (or shaded pink) of meat.[11] Very little nitrite is needed to induce this change.[11] It has been reported that as little as 2 to 14 parts per million (ppm) is needed to induce this desirable color change.[18] However, to extend the lifespan of this color change, significantly higher levels are needed.[18] The mechanism responsible for this color change is the formation of nitrosylating agents by nitrite, which has the ability to transfer nitric oxide that subsequently reacts with myoglobin to produce the cured meat color.[18] The unique taste associated with cured meat is also affected by the addition of sodium nitrite.[11] However, the mechanism underlying this change in taste is still not fully understood.[18]
Inhibition of microbial growth
A 2018 study by the British Meat Producers Association determined that legally permitted levels of nitrite have no effect on the growth of the Clostridium botulinum bacteria which causes botulism, in line with the UK's Advisory Committee on the Microbiological Safety of Food opinion that nitrites are not required to prevent C. botulinum growth and extend shelf life.[19] In some countries, cured-meat products are manufactured without nitrites. For example, Parma ham, which has been produced without nitrite since 1993, was reported in 2018 to have caused no cases of botulism.[7]
Sodium nitrite has shown varying degrees of effectiveness for controlling growth of other spoilage or disease causing microorganisms.[11] Although the inhibitory mechanisms are not well known, its effectiveness depends on several factors including residual nitrite level, pH, salt concentration, reductants present and iron content.[18] The type of bacteria also affects sodium nitrite's effectiveness.[18] It is generally agreed that sodium nitrite is not effective for controlling Gram-negative enteric pathogens such as Salmonella and Escherichia coli.[18]
Other food additives (such as lactate and sorbate) provide similar protection against bacteria, but do not provide the desired pink color.[20][21]
Inhibition of lipid peroxidation
Sodium nitrite is also able to effectively delay the development of oxidative rancidity.[18] Lipid peroxidation is considered to be a major reason for the deterioration of quality of meat products (rancidity and unappetizing flavors).[18] Sodium nitrite acts as an antioxidant in a mechanism similar to the one responsible for the coloring effect.[18] Nitrite reacts with heme proteins and metal ions, neutralizing free radicals by nitric oxide (one of its byproducts).[18] Neutralization of these free radicals terminates the cycle of lipid oxidation that leads to rancidity.[18]
Medication
 Chemical structure | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | FDA Professional Drug Information |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| E number | E250 (preservatives) |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.028.687 |
| Chemical and physical data | |
| Formula | NNaO2 |
| Molar mass | 68.995 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Sodium nitrite is used as a medication together with sodium thiosulfate to treat cyanide poisoning.[22] It is recommended only in severe cases of cyanide poisoning.[23] In those who have both cyanide poisoning and carbon monoxide poisoning sodium thiosulfate by itself is usually recommended.[24] It is given by slow injection into a vein.[22]
Side effects can include low blood pressure, headache, shortness of breath, loss of consciousness, and vomiting.[22] Greater care should be taken in people with underlying heart disease.[22] The patient's levels of methemoglobin should be regularly checked during treatment.[22] While not well studied during pregnancy, there is some evidence of potential harm to the baby.[25] Sodium nitrite is believed to work by creating methemoglobin that then binds with cyanide and thus removes it from the mitochondria.[25]
Sodium nitrite came into medical use in the 1920s and 1930s.[26][27] It is on the World Health Organization's List of Essential Medicines.[28]
Suicide
Several academic publications in 2020 and 2021 have discussed the toxicity of sodium nitrite, and an apparent recent increase in suicides from using sodium nitrite which had been ordered online.[29][30][31][32][33][34][35][36][37][38] The usage of sodium nitrite as a suicide method has been heavily discussed on suicide forums.[39] Sodium nitrite was also the culprit of the McCarthy et al. v Amazon lawsuit alleging that Amazon knowingly assisted in the deaths of healthy children by selling them "suicide kits" as Amazon's "frequently bought together" feature recommended buying sodium nitrite, an antiemetic and a suicide instruction book together.[40]
Toxicity
Sodium nitrite is toxic.[41] The LD50 in rats is 180 mg/kg and in human LDLo is 71 mg/kg.[42] Yet, death by sodium nitrite ingestion can happen at lower dose.[43][44] Sodium nitrite is sometimes used for homicide.[45][46] The online marketplace eBay has globally prohibited the sale of sodium nitrite since 2019.[47] To prevent accidental intoxication, sodium nitrite (blended with salt) sold as a food additive in the US is dyed bright pink to avoid mistaking it for plain salt or sugar. In other countries, nitrited curing salt is not dyed but is strictly regulated.[48]
Occurrence in vegetables
Nitrites are not naturally occurring in vegetables in significant quantities.[49] Boiling vegetables does not affect nitrite levels.[50]
The presence of nitrite in animal tissue is a consequence of metabolism of nitric oxide, an important neurotransmitter.[51] Nitric oxide can be created de novo from nitric oxide synthase utilizing arginine or from ingested nitrite.[52]
Pigs
Because of sodium nitrite's high level of toxicity to swine (Sus scrofa) it is now being developed in Australia to control feral pigs and wild boar.[53][54] The sodium nitrite induces methemoglobinemia in swine, i.e. it reduces the amount of oxygen that is released from hemoglobin, so the animal will feel faint and pass out, and then die in a humane manner after first being rendered unconscious.[55] The Texas Parks and Wildlife Department operates a research facility at Kerr Wildlife Management Area, where they examine feral pig feeding preferences and bait tactics to administer sodium nitrite.[56]
Cancer
Carcinogenicity is the ability or tendency of a chemical to induce tumors, increase their incidence or malignancy, or shorten the time of tumor occurrence.[57]
Adding nitrites to meat has been shown to generate known carcinogens such as nitrosamines; the World Health Organization (WHO) advises that each 50 g (1.8 oz) of "processed meats" eaten a day would raise the risk of getting bowel cancer by 18% over a lifetime. The World Health Organization's review of more than 400 studies concluded, in 2015, that there was sufficient evidence that "processed meats" caused cancer, particularly colon cancer;[7] the WHO's International Agency for Research on Cancer (IARC) classified "processed meats" as carcinogenic to humans (Group 1); "processed meat" meaning meat that has been transformed through salting, curing, fermentation, smoking, or other processes to enhance flavour or improve preservation.).[7][58]
Nitrosamines can be formed during the curing process used to preserve meats, when sodium nitrite-treated meat is cooked, and also from the reaction of nitrite with secondary amines under acidic conditions (such as occurs in the human stomach). Dietary sources of nitrosamines include US cured meats preserved with sodium nitrite as well as the dried salted fish eaten in Japan. In the 1920s, a significant change in US meat curing practices resulted in a 69% decrease in average nitrite content. This event preceded the beginning of a dramatic decline in gastric cancer mortality.[59] Around 1970, it was found that ascorbic acid (vitamin C), an antioxidant, inhibits nitrosamine formation.[60] Consequently, the addition of at least 550 ppm of ascorbic acid is required in meats manufactured in the United States. Manufacturers sometimes instead use erythorbic acid, a cheaper but equally effective isomer of ascorbic acid. Additionally, manufacturers may include α-tocopherol (vitamin E) to further inhibit nitrosamine production. α-Tocopherol, ascorbic acid, and erythorbic acid all inhibit nitrosamine production by their oxidation-reduction properties. Ascorbic acid, for example, forms dehydroascorbic acid when oxidized, which when in the presence of nitrosonium, a potent nitrosating agent formed from sodium nitrite, reduces the nitrosonium into nitric oxide.[61] The nitrosonium ion formed in acidic nitrite solutions is commonly[62][63] mislabeled nitrous anhydride, an unstable nitrogen oxide that cannot exist in vitro.[64]
Ingesting nitrite under conditions that result in endogenous nitrosation has been classified as "probably carcinogenic to humans" by International Agency for Research on Cancer (IARC).[65][66]
Sodium nitrite consumption has also been linked to the triggering of migraines in individuals who already experience them.[67]
One study has found a correlation between highly frequent ingestion of meats cured with pink salt and the COPD form of lung disease. The study's researchers suggest that the high amount of nitrites in the meats was responsible; however, the team did not prove the nitrite theory. Additionally, the study does not prove that nitrites or cured meat caused higher rates of COPD, merely a link. The researchers did adjust for many of COPD's risk factors, but they commented they cannot rule out all possible unmeasurable causes or risks for COPD.[68][69]
Production
Industrial production of sodium nitrite follows one of two processes, the reduction of nitrate salts, or the oxidation of lower nitrogen oxides.
One method uses molten sodium nitrate as the salt, and lead which is oxidized, while a more modern method uses scrap iron filings to reduce the nitrate.[4][70]
A more commonly used method involves the general reaction of nitrogen oxides in alkaline aqueous solution, with the addition of a catalyst. The exact conditions depend on which nitrogen oxides are used, and what the oxidant is, as the conditions need to be carefully controlled to avoid over oxidation of the nitrogen atom.[4]
Sodium nitrite has also been produced by reduction of nitrate salts by exposure to heat, light, ionizing radiation, metals, hydrogen, and electrolytic reduction.[71]
Chemical reactions
In the laboratory, sodium nitrite can be used to destroy excess sodium azide.[72][73]
Above 330 °C sodium nitrite decomposes (in air) to sodium oxide, nitric oxide and nitrogen dioxide.[74]
Sodium nitrite can also be used in the production of nitrous acid:
The nitrous acid then, under normal conditions, decomposes:
The resulting nitrogen dioxide hydrolyzes to a mixture of nitric and nitrous acids:
Isotope labelling 15N
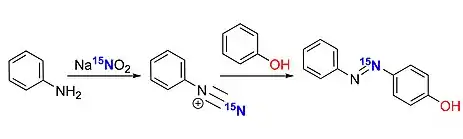
In organic synthesis isotope enriched sodium nitrite-15N can be used instead of normal sodium nitrite as their reactivity is nearly identical in most reactions.
The obtained products carry isotope 15N and hence Nitrogen NMR can be efficiently carried out.[75]
References
- Gohda T, Ichikawa M (November 1996). "The Refinement of the Structure of Ferroelectric Sodium Nitrite". Journal of the Korean Physical Society. 29: 551–554.
- Zumdahl SS (2009). Chemical Principles (6th ed.). Houghton Mifflin Company. p. A23. ISBN 978-0-618-94690-7.
- "GESTIS-Stoffdatenbank sodium nitrite". gestis.dguv.de. Retrieved 10 December 2021.
- Laue W, Thiemann M, Scheibler E, Wiegand KW (2006). "Nitrates and Nitrites". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_265.
- Krakhmalev SI, Vorotnikova VA, Ten NV, Taranova NV (1984). "Determination of sodium nitrite in complex sodium oils". Chemistry and Technology of Fuels and Oils. 20 (12): 612–613. doi:10.1007/BF00726438. S2CID 94383988.
- "Sodium Nitrite". General Chemical. Retrieved 28 September 2012.
- Wilson B (1 March 2018). "Yes, bacon really is killing us". The Guardian. London. ISSN 0261-3077. Archived from the original on 10 February 2021. Retrieved 14 February 2021.
In trade journals of the 1960s, the firms who sold nitrite powders to ham-makers spoke quite openly about how the main advantage was to increase profit margins by speeding up production.
- Lerfall J, Østerlie M (February 2011). "Use of sodium nitrite in salt-curing of Atlantic salmon (Salmo salar L.) – Impact on product quality". Food Chemistry. 124 (3): 759–766. doi:10.1016/j.foodchem.2010.06.092.
- Bailey ME, Frame RW, Naumann HD (January 1964). "Cured Meat Pigments, Studies of the Photooxidation of Nitrosomyoglobin". Journal of Agricultural and Food Chemistry. 12 (1): 89–93. doi:10.1021/jf60131a026.
- ""Meat Pigment Chemistry", taken from IFT Mini-Experiments in Food Science Series" (PDF).
- Sindelar JJ, Milkowski AL (May 2012). "Human safety controversies surrounding nitrate and nitrite in the diet". Nitric Oxide. 26 (4): 259–266. doi:10.1016/j.niox.2012.03.011. PMID 22487433.
- "Science Says: Are hot dogs healthier without added nitrites? | Lifestyle from CTV News". www.ctvnews.ca. 30 June 2017.
- UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 27 October 2011.
- "Health and Food Safety" (PDF). European Commission – European Commission. Retrieved 1 April 2018.
- US Food and Drug Administration: "Listing of Food Additives Status Part II". Food and Drug Administration. Retrieved 27 October 2011.
- Australia New Zealand Food Standards Code"Standard 1.2.4 – Labelling of ingredients". Retrieved 27 October 2011.
- Coudray G (February 2021). Who poisoned your bacon?. Icon Books. pp. xv. ISBN 978-1785786112.
- Sindelar J, Milkowski A (November 2011). "Sodium Nitrite in Processed Meat and Poultry Meats: A Review of Curing and Examining the Risk/Benefit of Its Use" (PDF). American Meat Science Association. 3: 1–14.
- Doward J (23 March 2019). "Revealed: no need to add cancer-risk nitrites to ham". The Observer. London. Archived from the original on 26 January 2021. Retrieved 14 February 2021.
The results show that there is no change in levels of inoculated C botulinum over the curing process, which implies that the action of nitrite during curing is not toxic to C botulinum spores at levels of 150 ppm [parts per million] ingoing nitrite and below.
- Seward RA, Deibel RH, Lindsay RC (November 1982). "Effects of potassium sorbate and other antibotulinal agents on germination and outgrowth of Clostridium botulinum type E spores in microcultures". Applied and Environmental Microbiology. 44 (5): 1212–1221. Bibcode:1982ApEnM..44.1212S. doi:10.1128/AEM.44.5.1212-1221.1982. PMC 242170. PMID 6758699.
- Sofos JN, Busta FF, Bhothipaksa K, Allen CE, Robach MC, Paquette MW (September 1980). "Effects of various concentrations of sodium nitrite and potassium sorbate on Clostridium botulinum toxin production in commercially prepared bacon". Journal of Food Science. 45 (5): 1285–1292. doi:10.1111/j.1365-2621.1980.tb06539.x.
- World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 65. hdl:10665/44053. ISBN 9789241547659.
- "Sodium Nitrite Solution for Injection - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. Archived from the original on 18 September 2017. Retrieved 15 January 2017.
- Baren JM (2008). Pediatric Emergency Medicine. Elsevier Health Sciences. p. 1018. ISBN 978-1416000877. Archived from the original on 16 January 2017.
- "Sodium Nitrite Injection - FDA prescribing information, side effects and uses". www.drugs.com. Archived from the original on 18 January 2017.
- Dart RC (2004). Medical Toxicology. Lippincott Williams & Wilkins. p. 172. ISBN 9780781728454. Archived from the original on 16 January 2017.
- Bryan NS, Loscalzo J (2011). Nitrite and Nitrate in Human Health and Disease. Springer Science & Business Media. p. 226. ISBN 9781607616160. Archived from the original on 16 January 2017.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Durão C, Pedrosa F, Dinis-Oliveira RJ (July 2020). "A fatal case by a suicide kit containing sodium nitrite ordered on the internet". Journal of Forensic and Legal Medicine. 73: 101989. doi:10.1016/j.jflm.2020.101989. hdl:10400.26/34138. PMID 32658747. S2CID 219909654.
- Durão C, Pedrosa F, Dinis-Oliveira RJ (June 2021). "Another suicide by sodium nitrite and multiple drugs: an alarming trend for "exit"?". Forensic Science, Medicine, and Pathology. 17 (2): 362–366. doi:10.1007/s12024-020-00340-2. PMID 33247411. S2CID 227180461.
- Tomsia M, Głaz M, Nowicka J, Szczepański M (July 2021). "Sodium nitrite detection in costal cartilage and vitreous humor - Case report of fatal poisoning with sodium nitrite". Journal of Forensic and Legal Medicine. 81: 102186. doi:10.1016/j.jflm.2021.102186. PMID 34058704. S2CID 235268052.
- McCann SD, Kennedy JM, Tweet MS, Bryant SM (March 2021). "Sodium Nitrite Ingestion: an Emerging Trend in Suicide Attempts Shared via Online Communities". The Journal of Emergency Medicine. 60 (3): 409–412. doi:10.1016/j.jemermed.2020.10.021. PMID 33712114. S2CID 232218908.
- Dean DE, Looman KB, Topmiller RG (July 2021). "Fatal methemoglobinemia in three suicidal sodium nitrite poisonings". Journal of Forensic Sciences. 66 (4): 1570–1576. doi:10.1111/1556-4029.14689. PMID 33598944. S2CID 231952466.
- Hickey TB, MacNeil JA, Hansmeyer C, Pickup MJ (September 2021). "Fatal methemoglobinemia: A case series highlighting a new trend in intentional sodium nitrite or sodium nitrate ingestion as a method of suicide". Forensic Science International. 326: 110907. doi:10.1016/j.forsciint.2021.110907. PMID 34298207.
- Harvey M, Cave G, Chanwai G (October 2010). "Fatal methaemoglobinaemia induced by self-poisoning with sodium nitrite". Emergency Medicine Australasia. 22 (5): 463–465. doi:10.1111/j.1742-6723.2010.01335.x. PMID 21040485. S2CID 38780081.
- McCann SD, Tweet MS, Wahl MS (December 2021). "Rising incidence and high mortality in intentional sodium nitrite exposures reported to US poison centers". Clinical Toxicology. 59 (12): 1264–1269. doi:10.1080/15563650.2021.1905162. PMID 33787434. S2CID 232431594.
- Sedhai YR, Atreya A, Basnyat S, Phuyal P, Pokhrel S (June 2022). "The use of sodium nitrite for deliberate self-harm, and the online suicide market: Should we care?". The Medico-Legal Journal. 90 (2): 79–80. doi:10.1177/0025817221998119. PMID 33906496. S2CID 233429578.
- Mudan A, Repplinger D, Lebin J, Lewis J, Vohra R, Smollin C (September 2020). "Severe Methemoglobinemia and Death From Intentional Sodium Nitrite Ingestions". The Journal of Emergency Medicine. 59 (3): e85–e88. doi:10.1016/j.jemermed.2020.06.031. PMID 32713620. S2CID 220797852.
- Twohey M (9 December 2021). "Where the Despairing Log On, and Learn Ways to Die". The New York Times. Retrieved 24 July 2022.
- ""Amazon "suicide kits" have led to teen deaths, according to new lawsuit"". Ars technica. 7 October 2022.
- Crellin J. "How toxic is it?" (PDF). The Association for Science Educators. Retrieved 6 February 2022.
- "Safety data for sodium nitrite". The Physical and Theoretical Chemistry Laboratory. Oxford University. Archived from the original on 10 April 2008.
- Gowans WJ (November 1990). "Fatal methaemoglobinaemia in a dental nurse. A case of sodium nitrite poisoning". The British Journal of General Practice. 40 (340): 470–471. PMC 1371420. PMID 2271282.
- Standefer JC, Jones AM, Street E, Inserra R (October 1979). "Death associated with nitrite ingestion: report of a case". Journal of Forensic Sciences. 24 (4): 768–771. doi:10.1520/JFS10905J. PMID 541641.
- "Chinese teacher sentenced to death for poisoning nursery children". BBC News. 29 September 2020.
- "Teacher in China sentenced to death for poisoning children's porridge". The Guardian. Agence France-Presse in Beijing. 29 September 2020.
- Director eBay (UK) Limited (8 January 2021). "Re Jason Thompson (deceased) – Sodium Nitrite" (PDF).
- "The Use and Removal of Nitrite in Meat Products | FAQs | the Food Safety Authority of Ireland".
- Dennis MJ, Wilson LA (2003). "Nitrates and Nitrites". Encyclopedia of Food Sciences and Nutrition. pp. 4136–4141. doi:10.1016/B0-12-227055-X/00830-0. ISBN 978-0-12-227055-0.
- Leszczyńska T, Filipiak-Florkiewicz A, Cieślik E, Sikora E, Pisulewski PM (June 2009). "Effects of some processing methods on nitrate and nitrite changes in cruciferous vegetables". Journal of Food Composition and Analysis. 22 (4): 315–321. doi:10.1016/j.jfca.2008.10.025.
- Meulemans A, Delsenne F (October 1994). "Measurement of nitrite and nitrate levels in biological samples by capillary electrophoresis". Journal of Chromatography. B, Biomedical Applications. 660 (2): 401–404. doi:10.1016/0378-4347(94)00310-6. PMID 7866533.
- Southan GJ, Srinivasan A (August 1998). "Nitrogen oxides and hydroxyguanidines: formation of donors of nitric and nitrous oxides and possible relevance to nitrous oxide formation by nitric oxide synthase". Nitric Oxide. 2 (4): 270–286. doi:10.1006/niox.1998.0187. PMID 9851368.
- Lapidge S, Wishart J, Smith M, Staples L (4 May 2009). Is America Ready for a Humane Feral Pig Toxicant?. Wildlife Damage Management Conference. Saratoga Springs, NY.
- WO 2008/104028, Cowled BD, Lapidge SJ, Humphrys S, Staples L, "Nitrite Salts as Poisons in Baits for Omnivores", published 2008
- Porter S, Kuchel T (2010). Assessing the humaness and efficacy of a new feral pig bait in domestic pigs. Study PC0409 (PDF). Canberra, South Australia: Veterinary Services Division, Institute of Medical and Veterinary Science. p. 11.
- Texas Parks and Wildlife (21 February 2013). "Hogs Wild – Fighting the Feral Pig Problem – Texas Parks and Wildlife [Official]". Archived from the original on 12 December 2021. Retrieved 1 April 2018 – via YouTube.
- "Known and Probable Human Carcinogens". www.cancer.org. Retrieved 28 January 2019.
- "IARC Monographs evaluate consumption of red meat and processed meat" (PDF). International Agency for Research on Cancer. 26 October 2015. Archived from the original (PDF) on 18 January 2021. Retrieved 14 February 2021.
Processed meat was classified as carcinogenic to humans (Group 1), based on sufficient evidence in humans that the consumption of processed meat causes colorectal cancer.
- Paik DC, Saborio DV, Oropeza R, Freeman HP (February 2001). "The epidemiological enigma of gastric cancer rates in the US: was grandmother's sausage the cause?". International Journal of Epidemiology. 30 (1): 181–182. doi:10.1093/ije/30.1.181. PMID 11171883.
- Mackerness CW, Leach SA, Thompson MH, Hill MJ (February 1989). "The inhibition of bacterially mediated N-nitrosation by vitamin C: relevance to the inhibition of endogenous N-nitrosation in the achlorhydric stomach". Carcinogenesis. 10 (2): 397–399. doi:10.1093/carcin/10.2.397. PMID 2492212.
- "Research Newsletter". Linus Pauling Institute. 1 July 2014. Retrieved 1 April 2018.
- Scanlan RA (May 1983). "Formation and occurrence of nitrosamines in food". Cancer Research. 43 (5 Suppl): 2435s–2440s. PMID 6831466. NAID 80001710206.
- Nollet LM, Toldrá F (2015). Handbook of Food Analysis (Third ed.). p. 290. ISBN 978-1-4822-9784-3.
- Williams DL (2004). "Reagents effecting nitrosation". Nitrosation Reactions and the Chemistry of Nitric Oxide. pp. 1–34. doi:10.1016/B978-044451721-0/50002-5. ISBN 978-0-444-51721-0.
- "List of classifications, Volumes 1–116 – IARC Monographs on the Evaluation of Carcinogenic Risks to Humans". International Agency for Research on Cancer (IARC) – World Health Organization (WHO). 2010. Retrieved 25 September 2016.
- Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. International Agency for Research on Cancer (IARC) – World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 94. 2010. ISBN 978-92-832-1294-2. Retrieved 25 September 2016.
- "Heading Off Migraine Pain". FDA Consumer magazine. U.S. Food and Drug Administration. 1998.
- Hitti M (17 April 2007). "Study: Cured Meats, COPD May Be Linked". WebMD Medical News.
- Jiang R, Paik DC, Hankinson JL, Barr RG (April 2007). "Cured meat consumption, lung function, and chronic obstructive pulmonary disease among United States adults". American Journal of Respiratory and Critical Care Medicine. 175 (8): 798–804. doi:10.1164/rccm.200607-969OC. PMC 1899290. PMID 17255565.
- Hao ZW, Xu XH, Wang DH (March 2005). "Reductive denitrification of nitrate by scrap iron filings". Journal of Zhejiang University. Science. B. 6 (3): 182–186. doi:10.1631/jzus.2005.B0182. PMC 1389719. PMID 15682502.
- Pokorny L, Maturana I, Bortle WH (2006). "Sodium Nitrate and Nitrite". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.1915040916151115.a01.pub2. ISBN 978-0-471-23896-6.
- "Sodium Azide". Hazardous Waste Management. Northeastern University. March 2003. Archived from the original on 4 November 2007.
- National Research Council (1995). Prudent practices in the laboratory: handling and disposal of chemicals. Washington, D.C.: National Academy Press. doi:10.17226/4911. ISBN 978-0-309-05229-0.
- Stern KH (July 1972). "High Temperature Properties and Decomposition of Inorganic Salts Part 3, Nitrates and Nitrites". Journal of Physical and Chemical Reference Data. 1 (3): 747–772. Bibcode:1972JPCRD...1..747S. doi:10.1063/1.3253104. S2CID 95532988.
- Kazem-Rostami M, Akhmedov NG, Faramarzi S (February 2019). "Molecular lambda shape light-driven dual switches: Spectroscopic and computational studies of the photoisomerization of bisazo Tröger base analogs". Journal of Molecular Structure. 1178: 538–543. Bibcode:2019JMoSt1178..538K. doi:10.1016/j.molstruc.2018.10.071. S2CID 105312344.
Further reading
- National Toxicology Program (May 2001). "Toxicology and carcinogenesis studies of sodium nitrite (CAS NO. 7632-00-0) in F344/N rats and B6C3F1 mice (drinking water studies)". National Toxicology Program Technical Report Series. 495: 7–273. PMID 12563346.
External links
- Drug information portal at the U.S. National Library of Medicine
- International Chemical Safety Card 1120.
- Nitrite in Meat
| HNO2 | He | ||||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)3 | C(NO2)4, CH(NO2)3, CH2(NO2)2, CH3(NO2) |
N(NO2)3 xNO3NO2 |
N2O3 | NO2F | Ne | ||||||||||||
| NaNO2 | Mg(NO2)2 | Al(NO2)3 | Si | P | S | NO2Cl | Ar | ||||||||||||
| KNO2 | Ca(NO2)2 | Sc(NO2)3 | Ti | VO(NO2)3 | Cr(NO2)3 | Mn(NO2)2 | Fe(NO2)3 | Co(NO2)2, Co(NO2)3 |
Ni(NO2)2 | Cu(NO2)2 | Zn(NO2)2 | Ga(NO2)3 | Ge | As | Se | NO2Br | Kr | ||
| RbNO2 | Sr(NO2)2 | Y(NO2)3 | Zr | Nb | Mo | Tc | Ru | Rh | Pd(NO2)2 | AgNO2 | Cd(NO2)2 | In | Sn | Sb | Te | NO2I | Xe | ||
| CsNO2 | Ba(NO2)2 | Hf | Ta | W | Re | Os | Ir | Pt(NO2)2, [Pt(NO2)4]2− |
Au | Hg2(NO2)2, Hg(NO2)2 |
TlNO2 | Pb(NO2)2 | Bi(NO2)3 BiO(NO2) |
Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(NO2)3 | Ce(NO2)3 | Pr(NO2)3 | Nd(NO2)3 | Pm | Sm(NO2)3 | Eu(NO2)2 | Gd(NO2)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac | Th | Pa | UO2(NO2)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||