Nalorphine
Nalorphine (INN) (brand names Lethidrone, Nalline), also known as N-allylnormorphine, is a mixed opioid agonist–antagonist with opioid antagonist and analgesic properties.[1] It was introduced in 1954[2] and was used as an antidote to reverse opioid overdose and in a challenge test to determine opioid dependence.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Lethidrone, Nalline |
| Other names | N-Allylnormorphine |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.497 |
| Chemical and physical data | |
| Formula | C19H21NO3 |
| Molar mass | 311.381 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Nalorphine was the second opioid antagonist to be introduced, preceded by nalodeine (N-allylnorcodeine) in 1915 and followed by naloxone in 1960 and naltrexone in 1963.[2] Due to potent activation of the κ-opioid receptor, nalorphine produces side effects such as dysphoria, anxiety, confusion, and hallucinations, and for this reason, is no longer used medically.[1][2][4]
Pharmacology
Pharmacodynamics
Nalorphine acts at two opioid receptors — the μ-opioid receptor (MOR) where it has antagonistic effects, and at the κ-opioid receptor (KOR) (Ki = 1.6 nM; EC50 = 483 nM; Emax = 95%) where it exerts high-efficacy partial agonist/near-full agonist characteristics.[5]
Chemistry
Analogues
Nalorphine has a number of analogues including niconalorphine (the nicomorphine analogue), diacetylnalorphine (heroin analogue), dihydronalorphine (dihydromorphine), and a number of others as well as a number of codeine-based analogues.[6]
Synthesis
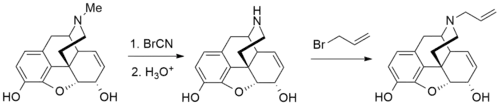
More recently, it has become much more commonplace to use ethyl chloroformate instead of cyanogen bromide for the Von Braun degradation demethylation step. See for example the list of phenyltropanes or the synthesis of paroxetine for further examples of this.
See also
- Diacetylnalorphine
- Levallorphan
- Nalbuphine
- Nalorphine dinicotinate
References
- Glatt M (6 December 2012). The Dependence Phenomenon. Springer Science & Business Media. pp. 121–. ISBN 978-94-011-7457-2.
- Aggrawal A. APC Essentials of Forensic Medicine and Toxicology. Avichal Publishing Company. pp. 554–. ISBN 978-81-7739-441-2.
- "Medicine: Drug Detector". Time. 24 December 1956. Archived from the original on July 26, 2005.
- Satoskar RS, Rege N, Bhandarkar SD (27 July 2015). Pharmacology and Pharmacotherapeutics. Elsevier Health Sciences APAC. pp. 166–. ISBN 978-81-312-4371-8.
- Gharagozlou P, Hashemi E, DeLorey TM, Clark JD, Lameh J (January 2006). "Pharmacological profiles of opioid ligands at kappa opioid receptors". BMC Pharmacology. 6 (1): 3. doi:10.1186/1471-2210-6-3. PMC 1403760. PMID 16433932.
- Casy AF, Parfitt RT (29 June 2013). Opioid Analgesics: Chemistry and Receptors. Springer Science & Business Media. ISBN 9781489905857 – via Google Books.
- McCawley EL, Hart ER, Marsh DF (January 1941). "The preparation of N-allylnormorphine". Journal of the American Chemical Society. 63 (1): 314. doi:10.1021/ja01846a504.
- Weijlard J, Erickson AE (1942). "N-Allylnormorphine". Journal of the American Chemical Society. 64 (4): 869–870. doi:10.1021/ja01256a036.
- Hart ER, McCawley EL (November 1944). "The pharmacology of N-allylnormorphine as compared with morphine". Journal of Pharmacology and Experimental Therapeutics. 82 (3): 339–48.
- U.S. Patent 2,364,833 (1944); Weijlard, U.S. Patent 2,891,954 (1959 to Merck & Co.).