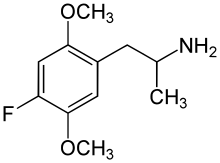
2,5-Dimethoxy-4-fluoroamphetamine
2,5-Dimethoxy-4-fluoroamphetamine (DOF) is a psychedelic drug of the phenethylamine and amphetamine classes. Alexander Shulgin briefly describes DOF in his book PiHKAL:[1]
Animal studies that have compared DOF to the highly potent DOI and DOB imply that the human activity will be some four to six times less than these two heavier halide analogues.[2]
— Alexander Shulgin, (PiHKAL)
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, Insufflation |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H16FNO2 |
| Molar mass | 213.252 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
DOF showed some stimulating effects in humans, but no psychedelic activity, after three doses of 6 mg spaced by one hour.[3] Daniel Trachsel further suspected that the molar refraction of the important 4-substituent in DOF and 2C-F may be too low to activate the 5-HT2A receptor sufficiently.[4] DOF more closely mimics the effects of the 4-unsubstituted 2,5-dimethoxyamphetamine than the effects of DOC, DOB, and DOI.[5][6][7]
See also
- 2,5-Dimethoxy-4-Substituted Amphetamines
References
- Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. United States: Transform Press. p. 978. ISBN 978-0-9630096-0-9.
- Glennon RA, Young R, Benington F, Morin RD (October 1982). "Behavioral and serotonin receptor properties of 4-substituted derivatives of the hallucinogen 1-(2,5-dimethoxyphenyl)-2-aminopropane". Journal of Medicinal Chemistry. 25 (10): 1163–8. doi:10.1021/jm00352a013. PMID 7143352.
- D.E. Nichols (1991). Biochemistry and Physiology of Substance Abuse, Vol. III. Boca Raton, Florida: CRC Press.
- Daniel Trachsel (July 2012). "Fluorine in psychedelic phenethylamines". Drug Testing and Analysis. 4 (7–8): 577–590. doi:10.1002/dta.413. PMID 22374819.
- David E. Nichols; Stewart Frescas; Danuta Marona-Lewicka; Xuemei Huang; Bryan L. Roth; Gary A. Gudelsky; J. Frank Nash (December 1994). "1-(2,5-Dimethoxy-4-(trifluoromethyl)phenyl)-2-aminopropane: a potent serotonin 5-HT2A/2C agonist". Journal of Medicinal Chemistry. 37 (25): 4346–4351. doi:10.1021/jm00051a011. PMID 7996545.
- Richard A. Glennon (1991). "Discriminative stimulus properties of hallucinogens and related designer drugs". NIDA Research Monograph. 116 (116): 25–44. PMID 1369672. Archived from the original on 2015-07-22. Retrieved 2015-06-29.
- Johnson MP; Hoffman AJ; Nichols DE; Mathis CA (December 1987). "Binding to the serotonin 5-HT2 receptor by the enantiomers of 125I-DOI". Neuropharmacology. 26 (12): 1803–1806. doi:10.1016/0028-3908(87)90138-9. PMID 3437942. S2CID 25077839.
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|