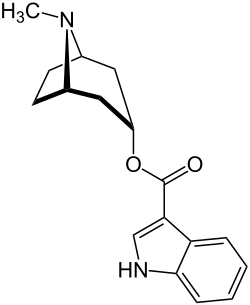
Tropisetron
Tropisetron is a serotonin 5-HT3 receptor antagonist used mainly as an antiemetic to treat nausea and vomiting following chemotherapy, although it has been used experimentally as an analgesic in cases of fibromyalgia.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Navoban |
| Other names | ICS 205-930 |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~60–80% |
| Protein binding | 71% |
| Metabolism | Hepatic (CYP3A4, CYP1A2, CYP2D6) |
| Elimination half-life | 6–8 hours |
| Excretion | Renal, Fecal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H20N2O2 |
| Molar mass | 284.359 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
It was patented in 1982 and approved for medical use in 1992.[2] It is on the World Health Organization's List of Essential Medicines.[3] It is marketed by Novartis in Europe, Australia, New Zealand, Japan, South Korea and the Philippines as Navoban, but is not available in the U.S. It is also available from Novell Pharmaceutical Laboratories and marketed in several Asian countries as Setrovel.
Pharmacology
Tropisetron acts as both a selective 5-HT3 receptor antagonist and α7-nicotinic receptor agonist.[4][5]
Adverse effects
Tropisetron is a well-tolerated drug with few side effects. Headache, constipation, and dizziness are the most commonly reported side effects associated with its use. Hypotension, transient liver enzyme elevation, immune hypersensitivity syndromes and extrapyramidal side effects have also been associated with its use on at least one occasion. There have been no significant drug interactions reported with this drug's use. It is broken down by the hepatic cytochrome P450 system and it has little effect on the metabolism of other drugs broken down by this system.
Other uses
As a biological stain and as trypanocide
See also
References
- Müller W, Stratz T (2004). "Local treatment of tendinopathies and myofascial pain syndromes with the 5-HT3 receptor antagonist tropisetron". Scandinavian Journal of Rheumatology. Supplement. 119 (119): 44–48. doi:10.1080/03009740410007032. PMID 15515413. S2CID 24916914.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 448. ISBN 9783527607495.
- World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, et al. (February 2001). "The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective alpha7 nicotinic receptor partial agonist". Bioorganic & Medicinal Chemistry Letters. 11 (3): 319–321. doi:10.1016/S0960-894X(00)00670-3. PMID 11212100.
- Cui R, Suemaru K, Li B, Kohnomi S, Araki H (May 2009). "Tropisetron attenuates naloxone-induced place aversion in single-dose morphine-treated rats: role of alpha7 nicotinic receptors". European Journal of Pharmacology. 609 (1–3): 74–77. doi:10.1016/j.ejphar.2008.12.051. PMID 19374878.