Laudexium metilsulfate
Laudexium metilsulfate is a neuromuscular blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in surgical anesthesia to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
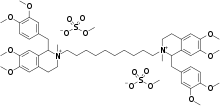 | |
| Clinical data | |
|---|---|
| Other names | Laudolissin |
| Routes of administration | IV |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C54H80N2O16S2 |
| Molar mass | 1077.35 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Laudexium[1] is no longer used in clinical practice, though it was introduced clinically in the early 1950s. It has about half the potency, a slower onset of action and a duration of action much longer than that of d-tubocurarine.[2] As with all clinically established (as well as experimental agents) with a non-depolarizing mechanism of action, its pharmacological action can be antagonized by anticholinesterases.
The displacement of laudexium from clinical use was assured owing to recurrent reports of significant post-operative re-curarization.[3]
References
- Taylor EP (1952). "Synthetic neuromuscular blocking agents. Part II. Bis(quaternary ammonium salts) derived from laudanosine". J Chem Soc: 142–145. doi:10.1039/JR9520000142.
- Hunter AR (February 1955). "The action of laudexium in man and experimental animals" (PDF). British Journal of Anaesthesia. 27 (2): 73–9. doi:10.1093/bja/27.2.73. PMID 13230365.
- Collier HO, Macauley B (September 1952). "The pharmacological properties of "laudolissin" a long-acting curarizing agent". British Journal of Pharmacology and Chemotherapy. 7 (3): 398–408. doi:10.1111/j.1476-5381.1952.tb00707.x. PMC 1509112. PMID 12978243.
External links
- Neuromuscular+blocking+agents at the US National Library of Medicine Medical Subject Headings (MeSH)