Mephenesin
Mephenesin (INN) is a centrally acting muscle relaxant. It can be used as an antidote for strychnine poisoning. Mephenesin however presents with the major drawbacks of having a short duration of action and a much greater effect on the spinal cord than the brain, resulting in pronounced respiratory depression at clinical doses and therefore a very low therapeutic index. It is especially dangerous and potentially fatal in combination with alcohol and other depressants.[1] Mephenesin was used by Bernard Ludwig and Frank Berger to synthesize meprobamate, the first tranquilizer to see widespread clinical use. Mephenesin is no longer available in North America but is used in Italy and a few other countries.[2] Its use has largely been replaced by the related drug methocarbamol, which is better absorbed.[3]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.389 |
| Chemical and physical data | |
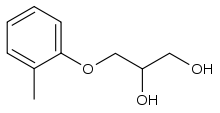
| Formula | C10H14O3 |
| Molar mass | 182.219 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Mephenesin may be an NMDA receptor antagonist.[4] Mephenesin was previously used in France as an OTC muscle relaxant called Décontractyl but was taken out of production by Sanofi Aventis and due to a French Health Ministry decree in July 2019. Mephenesin is, however, still available in Italy.
External links
- Bachmeyer C, Blum L, Fléchet M, Duriez P, Cabane J, Imbert J (1996). "[Severe contact dermatitis caused by mephenesin]". Ann Dermatol Venereol. 123 (3): 185–7. PMID 8761781.
- Ono H, Nakamura T, Ito H, Oka J, Fukuda H (1987). "Rigidity in rats due to radio frequency decerebration and effects of chlorpromazine and mephenesin". Gen Pharmacol. 18 (1): 57–9. doi:10.1016/0306-3623(87)90170-4. PMID 3557053.
References
- "Mephenesin". MIMS.
- "Mephenesin". Drugs.com.
- Huf, Ernst; et al. (1959). "Comparative Plasma Levels of Mephenesin, Mephenesin Carbamate and Methocarbamol". Proceedings of the Society for Experimental Biology and Medicine. Experimental Biology & Medicine. 102 (2): 276–7. doi:10.3181/00379727-102-25218. PMID 14403806. S2CID 37483102. Retrieved 8 January 2014.
- Keshavarz M, Showraki A, Emamghoreishi M (2013). "Anticonvulsant Effect of Guaifenesin against Pentylenetetrazol-Induced Seizure in Mice". Iran J Med Sci. 38 (2): 116–21. PMC 3700057. PMID 23825891.