Radafaxine
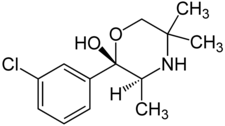
Radafaxine (developmental code name GW-353,162), also known as (2S,3S)-hydroxybupropion or (S,S)-hydroxybupropion,[1] is a norepinephrine–dopamine reuptake inhibitor (NDRI) which was under development by GlaxoSmithKline in the 2000s for a variety of different indications but was never marketed.[2] These uses included treatment of restless legs syndrome, major depressive disorder, bipolar disorder, neuropathic pain, fibromyalgia, and obesity.[2] Regulatory filing was planned for 2007,[3] but development was discontinued in 2006 due to "poor test results".[4]
 | |
| Clinical data | |
|---|---|
| Other names | (S,S)-Hydroxybupropion; (2S,3S)-Hydroxybupropion; GW-353,162 |
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| Chemical and physical data | |
| Formula | C13H18ClNO2 |
| Molar mass | 255.74 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Pharmacology
Pharmacodynamics
Radafaxine is described as a norepinephrine–dopamine reuptake inhibitor (NDRI). In contrast to bupropion, it appears to have a higher potency on inhibition of norepinephrine reuptake than on dopamine reuptake. Radafaxine has about 70% of the efficacy of bupropion in blocking dopamine reuptake, and 392% of efficacy in blocking norepinephrine reuptake, making it fairly selective for inhibiting the reuptake of norepinephrine over dopamine.[5][6] This, according to GlaxoSmithKline, may account for the increased effect of radafaxine on pain and fatigue.[7] At least one study suggests that radafaxine has a low abuse potential similar to bupropion.[8]
Chemistry
Radafaxine is a potent metabolite of bupropion, the compound in GlaxoSmithKline's Wellbutrin. More specifically, "hydroxybupropion" is an analogue of bupropion, and radafaxine is an isolated isomer ((2S,3S)-) of hydroxybupropion.[9] Therefore, radafaxine builds on at least some of the properties of bupropion in humans.[3] Another analogue of bupropion, manifaxine (GW-320,659), was derived from radafxine and was also studied.[10]
See also
References
- Carroll FI, Blough BE, Mascarella SW, Navarro HA, Lukas RJ, Damaj MI (2014). "Bupropion and bupropion analogs as treatments for CNS disorders". Emerging Targets & Therapeutics in the Treatment of Psychostimulant Abuse. Adv Pharmacol. Advances in Pharmacology. Vol. 69. pp. 177–216. doi:10.1016/B978-0-12-420118-7.00005-6. ISBN 9780124201187. PMID 24484978.
- "Radafaxine - AdisInsight".
- "Reviews Novel Therapeutics For CNS Disorders And Confirms Strong Pipeline Momentum". BioSpace. 23 November 2004. Archived from the original on 2007-09-28.
- Kollewe J (27 July 2006). "GSK breakthrough on bird flu vaccine". Independent.co.uk. Archived from the original on 2007-10-01.
- Xu H, Loboz KK, Gross AS, McLachlan AJ (March 2007). "Stereoselective analysis of hydroxybupropion and application to drug interaction studies". Chirality. 19 (3): 163–70. doi:10.1002/chir.20356. PMID 17167747.
- Bondarev ML, Bondareva TS, Young R, Glennon RA (August 2003). "Behavioral and biochemical investigations of bupropion metabolites". European Journal of Pharmacology. 474 (1): 85–93. doi:10.1016/S0014-2999(03)02010-7. PMID 12909199.
- Burch D. "Neurosciences Development Portfolio" (PDF). Archived from the original (PDF) on 2007-09-28.
- Volkow ND, Wang GJ, Fowler JS, Learned-Coughlin S, Yang J, Logan J, et al. (March 2005). "The slow and long-lasting blockade of dopamine transporters in human brain induced by the new antidepressant drug radafaxine predict poor reinforcing effects". Biological Psychiatry. 57 (6): 640–6. doi:10.1016/j.biopsych.2004.12.007. PMID 15780851. S2CID 13313064.
- Radafaxine at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Manifaxine - AdisInsight".