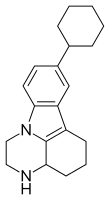
Tetrindole
Tetrindole was a drug candidate that functions by reversibly inhibiting monoamine oxidase A; it was first synthesized in Moscow in the early 1990s.[1] Tetrindole is similar in its chemical structure to pirlindole (Pyrazidol), and metralindole.[2]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C20H26N2 |
| Molar mass | 294.442 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Synthesis
The Fischer indole synthesis between 4-Cyclohexylphenylhydrazine [61624-13-3] (1) and 1,2-Cyclohexanedione [765-87-7] (2) gives 6-cyclohexyl-2,3,4,9-tetrahydrocarbazol-1-one [135897-70-0] (3).
There is 2 conceptual ways of continuing the synthesis. In the classical method chloroacetonitrile [107-14-2] (4) is used to alkylate the indole nitrogen to give 5. Reduction to the amine and subsequent imine formation, followed by reduction gives tetrindole. In the other method, ethanolamine is used instead.
References
- Medvedev AE, Kirkel AA, Kamyshanskaya NS, Moskvitina TA, Axenova LN, Gorkin VZ, et al. (January 1994). "Monoamine oxidase inhibition by novel antidepressant tetrindole". Biochemical Pharmacology. 47 (2): 303–8. doi:10.1016/0006-2952(94)90021-3. PMID 8304974.
- Ramsay RR, Gravestock MB (March 2003). "Monoamine oxidases: to inhibit or not to inhibit". Mini Reviews in Medicinal Chemistry. 3 (2): 129–36. doi:10.2174/1389557033405287. PMID 12570845.
- Vasily Ivanovich Shvedov, 18 More », WO 1991007968 (1991 to Vsesojuzny Nauchno-Issledovatelsky Khimiko-Farmatsevtichesky Inst. Imeni Sergo Ordzhonikidze (Vnikhfi)).
- Vasily Ivanovich Shvedov, et al. SU1694582 (1991).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
