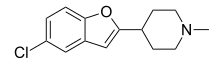
Sercloremine
Sercloremine (CGP-4718A), usually as the hydrochloride salt, is a drug which was developed in the 1980s and was formerly under investigation as an antidepressant, but was never marketed.[1][2] It acts as a selective, reversible inhibitor of monoamine oxidase A (RIMA) and serotonin reuptake inhibitor.[1][3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4-(5-Chloro-1-benzofuran-2-yl)-1-methylpiperidine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C14H16ClNO |
| Molar mass | 249.74 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
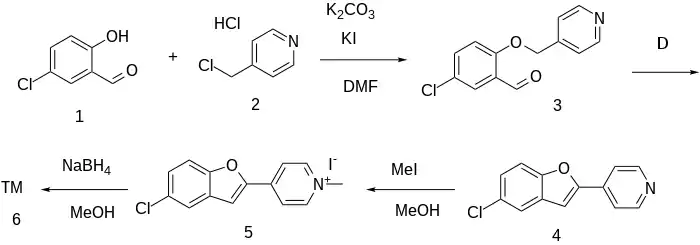
Ex 12: The ether formation between 5-chlorosalicylaldehyde [635-93-8] (1) and 4-(chloromethyl)-pyridine HCl [1822-51-1] (2) gives 2-[(4-pyridyl)-methoxy]-5-chlorobenzaldehyde CID:16769957 (3). Heating of this intermediate gives 4-(5-chloro-2-benzofuranyl)-pyridine CID:23312193 (4). Alkylation with methyl iodide leads to 1-methyl-4-(5-chloro-2-benzofuranyl)-pyridinium iodide, CID:23312242 (5). The reduction with sodium borohydride completes the synthesis of Sercloremine (6).
References
- Luttinger D, Hlasta DJ (January 1987). "Antidepressant agents". In Hesp B, Bailey DM (eds.). Annual Reports in Medicinal Chemistry. Vol. 22. Academic Press. pp. 21–30 (25). ISBN 978-0-08-058366-2.
- Ganellin CR, Triggle DJ (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1801–. ISBN 978-0-412-46630-4.
- Delini-Stula A, Fischbach R, Gnirss F, Bures E, Pöldinger W (1985). "Early experience with CGP 4718 A (Sercloremine), a new selective and reversible MAO-A and 5-HT-uptake inhibitor, in the treatment of depressive patients". Drug Development Research. 6 (4): 371–384. doi:10.1002/ddr.430060409. ISSN 0272-4391. S2CID 85113482.
- DE2408476 idem Karl Schenker, Raymond Bernasconi, U.S. Patent 4,210,655 (1980 to Ciba-Geigy Corporation).
- Karl Schenker & Raymond Bernasconi, U.S. Patent 4,600,719 (1986 to Novartis Corp).