Perafensine
Perafensine (INN) (code name HR-459) is a drug which was investigated as an antidepressant but was never marketed.[1] It has been reported to antagonize the effects of reserpine and to inhibit the reuptake of norepinephrine (norepinephrine reuptake inhibitor); whether it also affects the reuptake of serotonin or dopamine is unclear.[2]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H19N3 |
| Molar mass | 289.382 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Synthesis
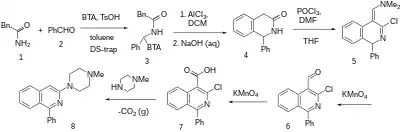
Treatment of 2-phenylacetamide [103-81-1] (1) with benzaldehyde (2) in the presence of benzotriazole gives the intermediate (3). A ring-closure can be achieved with aluminium chloride Lewis acid in DCM solvent. Quenching the reaction in aqueous lye then cleaves the BTA molecule giving a compound that is called 1-phenyl-1,2,4-trihydroisoquinolin-3-one, [17507-05-0] (4). A Vilsmeier-Haack reaction with phosphorus oxychloride and DMF in the presence of THF solvent gives 3-chloro-4-dimethylaminomethylene-1-phenyl isoquinoline, CID:53778830 (5). This intermediate is then oxidized with potassium permanganate in the presence of sulfuric acid to 3-chloro-1-phenyl-isoquinoline-4-aldehyde, [72118-68-4] (6). A more exhaustive second oxidation with potassium permanganate does on to give 3-chloro-1-phenyl-isoquinoline-4-carboxylic acid, CID:14567294 (7). The last step is the reaction with 1-methylpiperazine and decarboxylation completing the synthesis of (8).
See also
References
- Ganellin CR, Triggle DJ (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1585–. ISBN 978-0-412-46630-4.
- Bondinell W, Kaiser C (1982). Chapter 5. Antidepressants. Annual Reports in Medicinal Chemistry. Vol. 17. pp. 41–50. doi:10.1016/S0065-7743(08)60487-X. ISBN 9780120405176.
- Serradell MN, Castaner J (1982). "Perafensine". Drugs of the Future. JR Prous. 7: 580. doi:10.1358/dof.1982.007.08.1003843.
- US 4282222, Bartmann W, Konz E, Geyer HM, issued 1981, assigned to Hoechst Aktiengesellschaft.
- Katritzky AR, Lan X, Zhang Z (March 1993). "Novel routes to 1‐aryl‐1, 4‐dihydro‐3 (2H)‐isoquinolinones and 2‐substituted or 2, 3‐disubstituted benzofurans by intramolecular cyclizations". Journal of Heterocyclic Chemistry. 30 (2): 381–387. doi:10.1002/jhet.5570300216.
- US 4260763, Bartmann W, Konz E, Kruse HJ, issued 1981, assigned to Hoechst Aktiengesellschaft.
- Renger B, Konz E, Rüger W (1988). "Synthesis and Reactions of Isoquinoline Derivatives III. 1 Synthesis and Reactions of 3, 4-Dihalogeno-1-phenylisoquinolines". Synthesis. 1988 (9): 683–685. doi:10.1055/s-1988-27670.