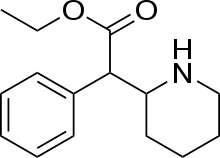
Ethylphenidate
Ethylphenidate (EPH), also known as Baxtercaine in the United Kingdom is a psychostimulant and a close analog of methylphenidate.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | EPH |
| Routes of administration | Insufflation, vaporized, intravenous, intramuscular, rectal, oral, sublingual |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Variable |
| Protein binding | Unknown |
| Metabolism | Hepatic transesterification of prodrugs methylphenidate and ethanol |
| Excretion | Urine, sweat |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H21NO2 |
| Molar mass | 247.338 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Ethylphenidate acts as both a dopamine reuptake inhibitor and norepinephrine reuptake inhibitor, meaning it effectively boosts the levels of the norepinephrine and dopamine neurotransmitters in the brain, by binding to, and partially blocking the transporter proteins that normally remove those monoamines from the synaptic cleft.
However, considering the close similarities between ethylphenidate and methylphenidate and the fact that methylphenidate, like cocaine, actually does not primarily act as a "classical" reuptake inhibitor, but rather as an "inverse agonist at the DAT" (also called a "negative allosteric modulator at the DAT"),[1] it is at least very likely that ethylphenidate also primarily acts as an inverse DAT agonist instead of (or at least only secondarily) as a classical reuptake inhibitor (which could be called a "competitive antagonist at the DAT" using a similar terminology as "negative allosteric modulator at the DAT", which per definition means that its mechanism is non-competitive).
Pharmacokinetics
Ethylphenidate metabolizes into methylphenidate and ritalinic acid.[2]
Tiny amounts of ethylphenidate can be formed in vivo when a solvent (specifically ethanol) and methylphenidate are coingested, via hepatic transesterification.[3] Ethylphenidate formation appears to be more common when large quantities of methylphenidate and alcohol are consumed at the same time, such as in non-medical use or overdose scenarios.[4] However, the transesterfication process of methylphenidate to ethylphenidate, as tested in mice liver, was dominant in the inactive (−)-enantiomer but showed a prolonged and increased maximal plasma concentration of the active (+)-enantiomer of methylphenidate.[5] Additionally, only a few percent of the consumed methylphenidate is converted to ethylphenidate.[3]
This carboxylesterase-dependent transesterification process is also known to occur when cocaine and alcohol are consumed together, forming cocaethylene.[6]
Pharmacodynamics
All available data on ethylphenidate's pharmacokinetics are drawn from studies conducted on rodents. Ethylphenidate is more selective to the dopamine transporter (DAT) than methylphenidate, having approximately the same efficacy as the parent compound,[5] but has significantly less activity on the norepinephrine transporter (NET).[7] Its dopaminergic pharmacodynamic profile is nearly identical to methylphenidate, and is primarily responsible for its euphoric and reinforcing effects.[8]
The eudysmic ratio for ethylphenidate is superior to that of methylphenidate.[5]
The following is ethylphenidate's binding profile in the mouse, alongside methylphenidate's. Figures for both the racemic and the dextrorotary enantiomers are given:[7]
| Compound | Binding DAT | Binding NET | Uptake DA | Uptake NE |
|---|---|---|---|---|
| d-methylphenidate | 139 | 408 | 28 | 46 |
| d-ethylphenidate | 276 | 2479 | 24 | 247 |
| dl-methylphenidate | 105 | 1560 | 24 | 31 |
| dl-ethylphenidate | 382 | 4824 | 82 | 408 |
Legality
- Ethylphenidate is not controlled internationally; see Convention on Psychotropic Substances
- Ethylphenidate is illegal in the Netherlands, as the Opium Law Lijst I covers it, as of 27 April 2018 [9]
- Ethylphenidate is not explicitly controlled in the US as part of the Controlled Substances Act but it could possibly be considered an analog of a Schedule II substance (methylphenidate) under the Federal Analog Act if sold for human consumption.
- Ethylphenidate is illegal in Sweden as of 15 December 2012.
- Ethylphenidate is illegal to manufacture, distribute or import in the UK, as of 10 April 2015 it has been placed under a Temporary Class Drug Order which automatically places it in a Class-B-like category.[10] Though ordinarily the TCDO would only last 1 year, the ACMD reported that since its invocation prevalence of MPA had significantly decreased, and that it had been challenging to collect information about the drug. As a result of this, they requested that the TCDO be extended a further year from 26 June 2016.[11]
- Ethylphenidate is illegal in Jersey under the Misuse of Drugs (Jersey) Law 1978.[12]
- Australian state and federal legislation contains provisions that mean that analogues of controlled drugs are also covered by the legislation. Ethylphenidate would be an analogue of methylphenidate under this legislation.[13]
- Ethylphenidate is controlled in Canada under the Controlled Drugs and Substances Act under Schedule III as of 05.05.2017.[14]
- Ethylphenidate is illegal in Germany as of 05.07.2013 [15]
- Ethylphenidate is illegal in Austria by the "Neue Psychoaktive Substanzen Gesetz" (=new psychoactive substances act) NPSG since 1 January 2012
- Ethylphenidate is illegal in Denmark as of 1 February 2013.[16]
- Ethylphenidate is illegal in Poland by "the Act on Counteracting Drug Addiction" since 1 July 2015 [17]
- It is illegal in Lithuania to use, buy, possess, transport, sell or import Ethylphenidate from 2015 [18]
- As of October 2015 Ethylphenidate is a controlled substance in China.[19]
See also
References
- Heal DJ, Gosden J, Smith SL (December 2014). "Dopamine reuptake transporter (DAT) "inverse agonism"--a novel hypothesis to explain the enigmatic pharmacology of cocaine". Neuropharmacology. 87: 19–40. doi:10.1016/j.neuropharm.2014.06.012. PMID 24953830. S2CID 4660652.
In vivo experiments in animals demonstrate that cocaine's monoaminergic pharmacology is profoundly different from that of other prescribed monoamine reuptake inhibitors, with the exception of methylphenidate. These findings led us to conclude that the highly unusual stimulant profile of cocaine and related compounds, eg methylphenidate, is not mediated by monoamine reuptake inhibition alone. We describe the experimental findings which suggest cocaine serves as a negative allosteric modulator to alter the function of the dopamine reuptake transporter (DAT) and reverse its direction of transport. This results in a firing-dependent, retro-transport of dopamine into the synaptic cleft. [...] Because the physiological role of DAT is to remove dopamine from the synapse and the action of cocaine is the opposite of this, we have postulated that cocaine's effect is analogous to an inverse agonist.
- Negreira N, Erratico C, van Nuijs AL, Covaci A (January 2016). "Identification of in vitro metabolites of ethylphenidate by liquid chromatography coupled to quadrupole time-of-flight mass spectrometry". Journal of Pharmaceutical and Biomedical Analysis. 117 (5): 474–84. doi:10.1016/j.jpba.2015.09.029. hdl:10067/1301870151162165141. PMID 26454340.
- Markowitz JS, DeVane CL, Boulton DW, Nahas Z, Risch SC, Diamond F, Patrick KS (June 2000). "Ethylphenidate formation in human subjects after the administration of a single dose of methylphenidate and ethanol". Drug Metabolism and Disposition. 28 (6): 620–4. PMID 10820132.
- Markowitz JS, Logan BK, Diamond F, Patrick KS (August 1999). "Detection of the novel metabolite ethylphenidate after methylphenidate overdose with alcohol coingestion". Journal of Clinical Psychopharmacology. 19 (4): 362–6. doi:10.1097/00004714-199908000-00013. PMID 10440465.
- Patrick KS, Williard RL, VanWert AL, Dowd JJ, Oatis JE, Middaugh LD (April 2005). "Synthesis and pharmacology of ethylphenidate enantiomers: the human transesterification metabolite of methylphenidate and ethanol". Journal of Medicinal Chemistry. 48 (8): 2876–81. doi:10.1021/jm0490989. PMID 15828826.
- Bourland JA, Martin DK, Mayersohn M (December 1997). "Carboxylesterase-mediated transesterification of meperidine (Demerol) and methylphenidate (Ritalin) in the presence of [2H6]ethanol: preliminary in vitro findings using a rat liver preparation". Journal of Pharmaceutical Sciences. 86 (12): 1494–6. doi:10.1021/js970072x. PMID 9423167.
- Williard RL, Middaugh LD, Zhu HJ, Patrick KS (February 2007). "Methylphenidate and its ethanol transesterification metabolite ethylphenidate: brain disposition, monoamine transporters and motor activity". Behavioural Pharmacology. 18 (1): 39–51. doi:10.1097/FBP.0b013e3280143226. PMID 17218796. S2CID 20232871.
- Jatlow P, Elsworth JD, Bradberry CW, Winger G, Taylor JR, Russell R, Roth RH (1991). "Cocaethylene: a neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion" (PDF). Life Sciences. 48 (18): 1787–94. doi:10.1016/0024-3205(91)90217-Y. hdl:2027.42/29671. PMID 2020260.
- Koninkrijksrelaties, Ministerie van Binnenlandse Zaken en. "Opiumwet". Wetten.overheid.nl. Retrieved 30 January 2022.
- "'Legal highs' to be banned under temporary power". Gov.uk.
- "Re: TCDOs and ACMD position on methylphenidate-based NPS" (PDF). Gov.uk. 2016-02-29. Retrieved 2016-11-28.
- "Archived copy" (PDF). Archived from the original (PDF) on 2014-05-03. Retrieved 2022-02-18.
{{cite web}}: CS1 maint: archived copy as title (link) - Parks, Claire; McKeown, Denise; Torrance, Hazel J. (2015-12-01). "A review of ethylphenidate in deaths in east and west Scotland". Forensic Science International. 257: 203–208. doi:10.1016/j.forsciint.2015.08.008. ISSN 0379-0738. PMID 26375622.
- Gazette, Government of Canada, Public Works and Government Services Canada, Public Services and Procurement Canada, Integrated Services Branch, Canada (2017-04-05). "Canada Gazette – Order Amending Schedule III to the Controlled Drugs and Substances Act (Methylphenidate)". Gazette.gc.ca.
- "BTMG - Einzelnorm". Archived from the original on 2013-10-16. Retrieved 2014-02-08.
- "Retsinformation". Retsinformation.dk. Retrieved 30 January 2022.
- "Ustawa z dnia 24 kwietnia 2015 r. o zmianie ustawy o przeciwdziałaniu narkomanii oraz niektórych innych ustaw". Isap.sejm.gov.pl.
- "Vartotojui - tik saugūs ir efektyvūs vaistai! - I SĄRAŠAS". Vvkt.lt.
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
See also
- Cocaethylene (compound formed when cocaine and ethanol are taken together)
- Methylphenidate
- Ethanol (drinking alcohol)