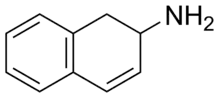
2-Amino-1,2-dihydronaphthalene
2-Amino-1,2-dihydronapthalene (2-ADN), also known as 2-aminodilin (2-AD), is a stimulant drug.[1] It is a rigid analogue of phenylisobutylamine and substitutes for amphetamine in rat discrimination tests, although at approximately one fourth the potency.[1] It is closely related to 2-aminotetralin (2-amino-1,2,3,4-tetrahydronaphthalene), which also substitutes for amphetamine, and is about two times as potent in comparison.[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H11N |
| Molar mass | 145.205 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
See also
- 2-Aminoindane
- 2-Aminotetralin
- 2-Naphthylamine
References
- Hathaway BA, Nichols DE, Nichols MB, Yim GK (May 1982). "A new, potent, conformationally restricted analogue of amphetamine: 2-amino-1,2-dihydronaphthalene". Journal of Medicinal Chemistry. 25 (5): 535–8. doi:10.1021/jm00347a011. PMID 6123601.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.