Tiflorex
Tiflorex, formerly known as flutiorex, is a stimulant amphetamine. Its most pronounced effect is in suppression of appetite; it has little effect on pulse rate, sleep, or mood.[1] It was found to be twice as potent an anorectic as fenfluramine.[2]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| Chemical and physical data | |
| Formula | C12H16F3NS |
| Molar mass | 263.32 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| | |
SL 72.340-d was cited to be 4x the anorectant potency of fenfluramine (ED50=1.4 mg/kg vs 5.6 mg/kg).[3]
Synthesis
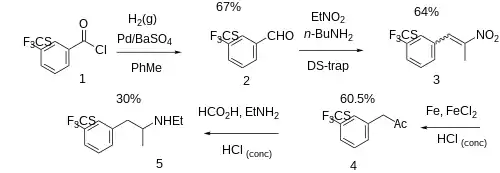
The Rosenmund reduction of 3-(trifluoromethylthio)benzoyl chloride [51748-28-8] (1) gave 3-((trifluoromethyl)thio)benzaldehyde [51748-27-7] (2). Henry reaction with nitroethane led to 1-(2-nitroprop-1-en-1-yl)-3-[(trifluoromethyl)sulfanyl]benzene [176242-84-5] (3). With the aid of iron catalyst in concentrated HCl acid there occurred FGI into 1-(3'-trifluoromethylthiophenyl)-2-propanone, CID:21325269 (4'). Reductive amination with ethylamine and formic acid as the reductant completed the synthesis of tiflorex (5).
References
- Silverstone T, Fincham J, Plumley J (April 1979). "An evaluation of the anorectic activity in man of a sustained release formulation of tiflorex". British Journal of Clinical Pharmacology. 7 (4): 353–6. doi:10.1111/j.1365-2125.1979.tb00945.x. PMC 1429648. PMID 444355.
- Giudicelli JF, Richer C, Berdeaux A (February 1976). "Preliminary assessment of flutiorex, a new anorectic drug, in man". British Journal of Clinical Pharmacology. 3 (1): 113–21. doi:10.1111/j.1365-2125.1976.tb00578.x. PMC 1428817. PMID 788737.
- Stuart S (2013-09-11). Abstracts: Sixth International Congress of Pharmacology. ISBN 9781483152530.
- Don P. R. L. Giudicelli & Henry Najer, U.S. Patent 4,148,923 (1979 to Synthelabo SA).
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|