Phenylpropanolamine
Phenylpropanolamine (PPA) is a sympathomimetic agent which is used as a decongestant and appetite suppressant.[3][1][4] It was commonly used in prescription and over-the-counter cough and cold preparations. In veterinary medicine, it is used to control urinary incontinence in dogs.[5][6]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Many[1][2] |
| AHFS/Drugs.com | Multum Consumer Information |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP2D6) |
| Elimination half-life | 2.1–3.4 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.035.349 |
| Chemical and physical data | |
| Formula | C9H13NO |
| Molar mass | 151.209 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Chemistry

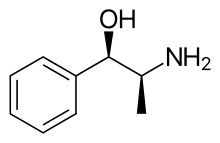
PPA is also known as β-hydroxyamphetamine, and is a member of the phenethylamine and amphetamine chemical classes.[3][7][8] It is closely related to the cathinones (β-ketoamphetamines).[7] The compound exists as four stereoisomers, which include d- and l-norephedrine and d- and l-norpseudoephedrine.[8][4] d-Norpseudoephedrine is also known as cathine,[3][8] and is found naturally in Catha edulis (khat).[9] Pharmaceutical drug preparations of PPA have varied in their stereoisomer composition in different countries, which may explain differences in misuse and side effect profiles.[4] Analogues of PPA include ephedrine, pseudoephedrine, amphetamine, methamphetamine, and cathinone.[7]
PPA, structurally, is in the substituted phenethylamine class, consisting of a cyclic benzene or phenyl group, a two carbon ethyl moiety, and a terminal nitrogen, hence the name phen-ethyl-amine.[10] The methyl group on the alpha carbon (the first carbon before the nitrogen group) also makes this compound a member of the substituted amphetamine class.[10] Ephedrine is the N-methyl analogue of PPA.
Exogenous compounds in this family are degraded too rapidly by monoamine oxidase to be active at all but the highest doses.[10] However, the addition of the α-methyl group allows the compound to avoid metabolism and confer an effect.[10] In general, N-methylation of primary amines increases their potency; whereas β-hydroxylation decreases CNS activity, but conveys more selectivity for adrenergic receptors.[10]
History
Phenylpropanolamine was patented in 1938.[11] In the United States, PPA is no longer sold due to an increased risk of haemorrhagic stroke.[12] In a few countries in Europe, however, it is still available either by prescription or sometimes over-the-counter. In Canada, it was withdrawn from the market on 31 May 2001.[13] It was voluntarily withdrawn from the Australian market by July 2001.[14] In India, human use of PPA and its formulations was banned on 10 February 2011,[15] but the ban was overturned by the judiciary in September 2011.[16]
Pharmacology
Mechanism of action
Although originally thought to act as a direct agonist of adrenergic receptors, PPA was subsequently found to show only weak or negligible affinity for these receptors, and has been instead characterized as an indirect sympathomimetic[4] which acts by inducing norepinephrine release and thereby activating adrenergic receptors.[17]
Pharmacodynamics
PPA acts primarily as a selective norepinephrine releasing agent.[17] It also acts as a dopamine releasing agent with around 10-fold lower potency.[17] The stereoisomers of the drug have only weak or negligible affinity for α- and β-adrenergic receptors.[17]
Many sympathetic hormones and neurotransmitters are based on the phenethylamine skeleton, and function generally in "fight or flight" type responses, such as increasing heart rate, blood pressure, dilating the pupils, increased energy, drying of mucous membranes, increased sweating, and a significant number of additional effects.
Activity profiles of isomers
| Compound | NE | DA | 5-HT |
|---|---|---|---|
| Norephedrine | |||
| D-Norephedrine | 42.1 | 302 | >10000 |
| L-Norephedrine (phenylpropanolamine) | 137 | 1371 | >10000 |
| Norpseudoephedrine | |||
| D-Norpseudoephedrine (cathine) | 15.0 | 68.3 | >10000 |
| L-Norpseudoephedrine | 30.1 | 294 | >10000 |
Pharmacokinetics
Norephedrine is a metabolite of amphetamine, as shown below.
Metabolic pathways of amphetamine in humans[sources 1]
|
Drug interactions
Certain drugs increase the chances of déjà vu occurring in the user, resulting in a strong sensation that an event or experience currently being experienced has already been experienced in the past. Some pharmaceutical drugs, when taken together, have also been implicated in the cause of déjà vu. Taiminen and Jääskeläinen (2001)[30] reported the case of an otherwise healthy male who started experiencing intense and recurrent sensations of déjà vu upon taking the drugs amantadine and phenylpropanolamine together to relieve flu symptoms. He found the experience so interesting that he completed the full course of his treatment and reported it to the psychologists to write up as a case study. Because of the dopaminergic action of the drugs and previous findings from electrode stimulation of the brain (e.g. Bancaud, Brunet-Bourgin, Chauvel, & Halgren, 1994),[31] Taiminen and Jääskeläinen speculate that déjà vu occurs as a result of hyperdopaminergic action in the mesial temporal areas of the brain.
Society and culture
Legal status
In Sweden, PPA is still available in prescription decongestants,[32] PPA is also still available in Germany. It is used in some polypill medications like Wick DayMed capsules.
In the United Kingdom, PPA was available in many 'all in one' cough and cold medications which usually also feature paracetamol or another analgesic and caffeine and could also be purchased on its own; however, it is no longer approved for human use. A European Category 1 Licence is required to purchase PPA for academic use.
In the United States, the Food and Drug Administration (FDA) issued a public health advisory[33] against the use of the drug in November 2000. In this advisory, the FDA requested that all drug companies discontinue marketing products containing PPA. The agency estimates that PPA caused between 200 and 500 strokes per year among 18-to-49-year-old users. In 2005, the FDA removed PPA from over-the-counter sale.[34] Because of its potential use in amphetamine manufacture, it is controlled by the Combat Methamphetamine Epidemic Act of 2005. It is still available for veterinary use in dogs, however, as a treatment for urinary incontinence.
Internationally, an item on the agenda of the 2000 Commission on Narcotic Drugs session called for including the stereoisomer norephedrine in Table I of United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.[35]
Drugs containing PPA were banned in India on 27 January 2011.[36] On 13 September 2011, Madras High Court revoked a ban on manufacture and sale of pediatric drugs PPA and nimesulide.[37]
Notes
- 4-Hydroxyamphetamine has been shown to be metabolized into 4-hydroxynorephedrine by dopamine beta-hydroxylase (DBH) in vitro and it is presumed to be metabolized similarly in vivo.[20][25] Evidence from studies that measured the effect of serum DBH concentrations on 4-hydroxyamphetamine metabolism in humans suggests that a different enzyme may mediate the conversion of 4-hydroxyamphetamine to 4-hydroxynorephedrine;[25][27] however, other evidence from animal studies suggests that this reaction is catalyzed by DBH in synaptic vesicles within noradrenergic neurons in the brain.[28][29]
References
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 828–. ISBN 978-3-88763-075-1.
- "Phenylpropanolamine Uses, Side Effects & Warnings".
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 70–71. ISBN 978-1-4757-2085-3.
- Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 219–. ISBN 978-94-011-4439-1.
- Gupta RC (23 April 2012). Veterinary Toxicology: Basic and Clinical Principles. Academic Press. pp. 458–. ISBN 978-0-12-385927-3.
- Riviere JE, Papich MG (17 March 2009). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. pp. 1309–. ISBN 978-0-8138-2061-3.
- Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 643–. ISBN 978-0-7817-6879-5.
- King LA (2009). Forensic Chemistry of Substance Misuse: A Guide to Drug Control. Royal Society of Chemistry. pp. 53–. ISBN 978-0-85404-178-7.
- Balint EE, Falkay G, Balint GA (2009). "Khat – a controversial plant". Wien. Klin. Wochenschr. 121 (19–20): 604–14. doi:10.1007/s00508-009-1259-7. PMID 19921126. S2CID 22816940.
- Westfall DP, Westfall TC (2010). "Chapter 12: Adrenergic Agonists and Antagonists: CLASSIFICATION OF SYMPATHOMIMETIC DRUGS". In Brunton LL, Chabner BA, Knollmann BC (eds.). Goodman & Gilman's Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill. ISBN 9780071624428.
CHEMISTRY AND STRUCTURE-ACTIVITY RELATIONSHIP OF SYMPATHOMIMETIC AMINES
β-Phenylethylamine (Table 12–1) can be viewed as the parent compound of the sympathomimetic amines, consisting of a benzene ring and an ethylamine side chain. The structure permits substitutions to be made on the aromatic ring, the α- and β-carbon atoms, and the terminal amino group to yield a variety of compounds with sympathomimetic activity. ...N-methylation increases the potency of primary amines ...
Substitution on the α-Carbon Atom
This substitution blocks oxidation by MAO, greatly prolonging the duration of action of non-catecholamines because their degradation depends largely on the action of this enzyme. The duration of action of drugs such as ephedrine or amphetamine is thus measured in hours rather than in minutes. Similarly, compounds with an α-methyl substituent persist in the nerve terminals and are more likely to release NE from storage sites. Agents such as metaraminol exhibit a greater degree of indirect sympathomimetic activity.
Substitution on the β-Carbon Atom
Substitution of a hydroxyl group on the β carbon generally decreases actions within the CNS, largely because it lowers lipid solubility. However, such substitution greatly enhances agonist activity at both α- and β- adrenergic receptors. Although ephedrine is less potent than methamphetamine as a central stimulant, it is more powerful in dilating bronchioles and increasing blood pressure and heart rate. - Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 541. ISBN 9783527607495.
- Yoon, et al. (2007). "Phenylpropanolamine contained in cold remedies and risk of hemorrhagic stroke". Neurology. 9 (68): 146–149. doi:10.1212/01.wnl.0000250351.38999.f2. PMID 17210897. S2CID 211233331. Retrieved 30 September 2020.
- "Advisories, Warnings and Recalls – 2001". Health Canada. 7 January 2009. Archived from the original on 3 May 2010.
- "Alert Phenylpropanolamine". Therapeutic Goods Administration. 7 March 2006. Retrieved 31 December 2018.
- "Drugs Banned In India". Dte.GHS, Ministry of Health and Family Welfare, Government of India. Central Drugs Standard Control Organization. Archived from the original on 13 October 2013. Retrieved 7 January 2014.
- "Madras High Court revokes ban on manufacture and sale of paediatric drugs nimesulide and PPA – India Medical Times".
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA (2003). "In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates". J. Pharmacol. Exp. Ther. 307 (1): 138–45. doi:10.1124/jpet.103.053975. PMID 12954796. S2CID 19015584.
- Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Current Topics in Medicinal Chemistry. 6 (17): 1845–59. doi:10.2174/156802606778249766. PMID 17017961.
- "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved 30 December 2013.
- Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39). ... The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). Journal of Biological Chemistry. 249 (2): 454–458. PMID 4809526. Retrieved 6 November 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacology & Therapeutics. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO - Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". Journal of Pharmacology and Experimental Therapeutics. 288 (3): 1251–1260. PMID 10027866.
- Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". Journal of Pharmaceutical and Biomedical Analysis. 30 (2): 247–255. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- Sjoerdsma A, von Studnitz W (April 1963). "Dopamine-beta-oxidase activity in man, using hydroxyamphetamine as substrate". British Journal of Pharmacology and Chemotherapy. 20: 278–284. doi:10.1111/j.1476-5381.1963.tb01467.x. PMC 1703637. PMID 13977820.
Hydroxyamphetamine was administered orally to five human subjects ... Since conversion of hydroxyamphetamine to hydroxynorephedrine occurs in vitro by the action of dopamine-β-oxidase, a simple method is suggested for measuring the activity of this enzyme and the effect of its inhibitors in man. ... The lack of effect of administration of neomycin to one patient indicates that the hydroxylation occurs in body tissues. ... a major portion of the β-hydroxylation of hydroxyamphetamine occurs in non-adrenal tissue. Unfortunately, at the present time one cannot be completely certain that the hydroxylation of hydroxyamphetamine in vivo is accomplished by the same enzyme which converts dopamine to noradrenaline.
- Badenhorst CP, van der Sluis R, Erasmus E, van Dijk AA (September 2013). "Glycine conjugation: importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation". Expert Opinion on Drug Metabolism & Toxicology. 9 (9): 1139–1153. doi:10.1517/17425255.2013.796929. PMID 23650932.
Figure 1. Glycine conjugation of benzoic acid. The glycine conjugation pathway consists of two steps. First benzoate is ligated to CoASH to form the high-energy benzoyl-CoA thioester. This reaction is catalyzed by the HXM-A and HXM-B medium-chain acid:CoA ligases and requires energy in the form of ATP. ... The benzoyl-CoA is then conjugated to glycine by GLYAT to form hippuric acid, releasing CoASH. In addition to the factors listed in the boxes, the levels of ATP, CoASH, and glycine may influence the overall rate of the glycine conjugation pathway.
- Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity". Circulation Research. 32 (5): 594–599. doi:10.1161/01.RES.32.5.594. PMID 4713201.
The biologic significance of the different levels of serum DβH activity was studied in two ways. First, in vivo ability to β-hydroxylate the synthetic substrate hydroxyamphetamine was compared in two subjects with low serum DβH activity and two subjects with average activity. ... In one study, hydroxyamphetamine (Paredrine), a synthetic substrate for DβH, was administered to subjects with either low or average levels of serum DβH activity. The percent of the drug hydroxylated to hydroxynorephedrine was comparable in all subjects (6.5-9.62) (Table 3).
- Freeman JJ, Sulser F (December 1974). "Formation of p-hydroxynorephedrine in brain following intraventricular administration of p-hydroxyamphetamine". Neuropharmacology. 13 (12): 1187–1190. doi:10.1016/0028-3908(74)90069-0. PMID 4457764.
In species where aromatic hydroxylation of amphetamine is the major metabolic pathway, p-hydroxyamphetamine (POH) and p-hydroxynorephedrine (PHN) may contribute to the pharmacological profile of the parent drug. ... The location of the p-hydroxylation and β-hydroxylation reactions is important in species where aromatic hydroxylation of amphetamine is the predominant pathway of metabolism. Following systemic administration of amphetamine to rats, POH has been found in urine and in plasma.
The observed lack of a significant accumulation of PHN in brain following the intraventricular administration of (+)-amphetamine and the formation of appreciable amounts of PHN from (+)-POH in brain tissue in vivo supports the view that the aromatic hydroxylation of amphetamine following its systemic administration occurs predominantly in the periphery, and that POH is then transported through the blood-brain barrier, taken up by noradrenergic neurones in brain where (+)-POH is converted in the storage vesicles by dopamine β-hydroxylase to PHN. - Matsuda LA, Hanson GR, Gibb JW (December 1989). "Neurochemical effects of amphetamine metabolites on central dopaminergic and serotonergic systems". Journal of Pharmacology and Experimental Therapeutics. 251 (3): 901–908. PMID 2600821.
The metabolism of p-OHA to p-OHNor is well documented and dopamine-β hydroxylase present in noradrenergic neurons could easily convert p-OHA to p-OHNor after intraventricular administration.
- Taiminen T, Jääskeläinen SK (September 2001). "Intense and recurrent déjà vu experiences related to amantadine and phenylpropanolamine in a healthy male". Journal of Clinical Neuroscience. 8 (5): 460–2. doi:10.1054/jocn.2000.0810. PMID 11535020. S2CID 6733989.
- Bancaud J, Brunet-Bourgin F, Chauvel P, Halgren E (February 1994). "Anatomical origin of déjà vu and vivid 'memories' in human temporal lobe epilepsy". Brain. 117 (1): 71–90. doi:10.1093/brain/117.1.71. PMID 8149215.
- "Rinexin in Farmaceutiska Specialiteter i Sverige" ["Rinexin" from the Pharmaceutical Specialties of Sweden] (drug catalog) (in Swedish). Retrieved 7 January 2014.
- "Phenylpopanolamine Advisory" (Press release). US Food and Drug Administration. 6 November 2000. Archived from the original on 26 January 2010.
- "Phenylpropanolamine (PPA) Information Page – FDA moves PPA from OTC" (Press release). US Food and Drug Administration. 23 December 2005. Archived from the original on 12 January 2009.
- Implementation of the international drug control treaties: changes in the scope of control of substances. Vienna: Commission on Narcotic Drugs, Forty-third session. 6–15 March 2000. Archived from the original on 14 August 2003.
- "Unsafe Drugs- nimesulide, Cisapride, Phenylpropanolamine Banned". 27 January 2011.
- "Madras High Court Revokes Ban on Manufacture and Sale PPA". Scribd.com. 13 September 2011. Retrieved 7 January 2014.
External links
- Phenylpropanolamine Information Page at FDA.gov (update, includes earlier reports)
- U.S. National Library of Medicine: Drug Information Portal – Phenylpropanolamine
