Talipexole
Talipexole (B-HT920, Domnin) is a dopamine agonist that is marketed as a treatment for Parkinson's Disease in Japan by Boehringer Ingelheim; it was introduced in 1996.[1] As of December 2014 it was not approved for marketing in the US nor in Europe.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Domin |
| Other names | Alefexole |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H15N3S |
| Molar mass | 209.31 g·mol−1 |
| 3D model (JSmol) |
|
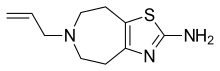
SMILES
| |
InChI
| |
| | |
Talipexole is a D2 dopamine receptor agonist and interacts both pre- and post-synaptic receptors. It also is an α2-adrenergic agonist.[3]
The main side effects are drowsiness, dizziness, hallucinations and minor gastrointestinal complaints.[3] In 2008 the Japanese Ministry of Health, Labour, and Welfare mandated that Boehringer add a warning to the label concerning the risk of sudden onset of sleep.[4]: 15
Synthesis
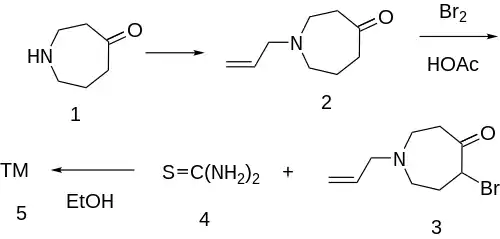
The N-alkylation of azepan-4-one [105416-56-6] (1) with allyl bromide in the presence of potassium carbonate gives 1-allyl-azepan-4-one (2). This is halogenated with molecular bromine in acetic acid to give 1-allyl-5-bromohexahydro-4-azepinone (3). The last step involves cyclization with thiourea (4) in refluxing ethanol, completing the synthesis of talipexole (5).
See also
- B-HT-958 [83718-64-3]
- Azepexole [36067-73-9]
- Pramipexole
References
- PharmaLetter 22 July 1996 First Launch In Japan For Talipexole
- EvaluatePharma Database. Page accessed 9 December 2014
- Benkert O, Müller-Siecheneder F, Wetzel H (1995). "Dopamine agonists in schizophrenia: a review". European Neuropsychopharmacology. 5 Suppl: 43–53. doi:10.1016/0924-977x(95)00022-h. PMID 8775758. S2CID 1600286.
- Japanese Ministry of Health, Labour and Welfare March 2008 Pharmaceuticals and Medical Devices Safety Information No. 245
- Serradell, M.N.; Blancafort, P.; Castaner, J.; Thorpe, P.J. Drugs Fut 1980,5(10),481.
- Gerhart Dipl-Chem Dr Griss, 3 More » idem, DE 2040510 (1972 to Thomae Gmbh Dr K).
- G Griss, M Kleemann, W Grell, H Ballhause, U.S. Patent 3,804,849 (1974 to Boehringer Sohn Ingelheim
- Deng Xianglin, et al. CN 104031072 (2014 to Chongqing Zen Pharmaceutical Co Ltd.).