Fluotracen
Fluotracen (SKF-28,175) is a tricyclic drug which possesses dual antidepressant and antipsychotic activity.[1][2][3] This profile of effects is similar to that of related agents like amoxapine, loxapine, and trimipramine which may also be used in the treatment of both depression and psychosis.[1] It was believed that such duality would be advantageous in the treatment of schizophrenia, as depression is often comorbid with the disorder and usual antipsychotics often worsen such symptoms.[1] In any case, however, fluotracen was never marketed.[3]
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H24F3N |
| Molar mass | 347.425 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Fluotracen is a rapidly acting antidepressant with clinical effects apparent often in 4 days. The maximum dosage was 200 mg/day. The mean effective dosage was 100 mg/day.[4]
Synthesis
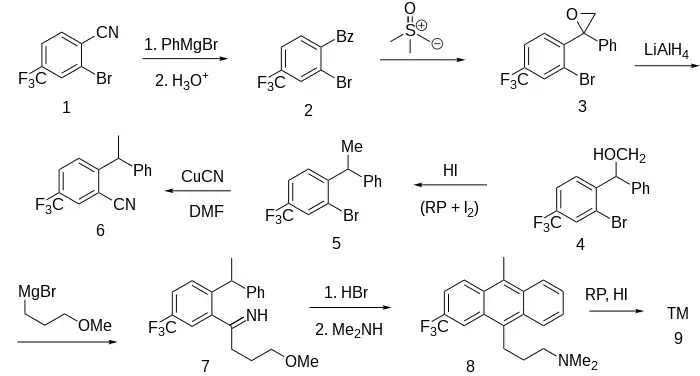
Ex 3: The Grignard reaction between 2-bromo-4-trifluoromethylbenzonitrile [35764-15-9] (1) and phenylmagnesium bromide gives, after hydrolysis, the benzophenone, [2-bromo-4-(trifluoromethyl)phenyl]-phenylmethanone, CID:154084654 (2).
Ex 4: Reaction of the ketone with the ylide from trimethylsulfoxonium iodide [1774-47-6] (Johnson–Corey–Chaykovsky reaction) leads to the epoxide, 2-[2-bromo-4-(trifluoromethyl)phenyl]-2-phenyloxirane, CID:154086694 (3). The reduction to the primary alcohol with lithium aluminium hydride gives (4). Further reduction by means of phosphorus and hydriodic acid completes conversion of the carbonyl to the homologous methyl group, 2-bromo-1-(1-phenylethyl)-4-(trifluoromethyl)benzene, CID:154127341 (5). Treatment with cuprous cyanide gives 2-(1-phenylethyl)-5-(trifluoromethyl)benzonitrile, CID:154134069 (6). Grignard reaction with 3-methoxybromopropane [36865-41-5] affords (7). Treatment of this intermediate with hydrobromic acid achieves both Friedel-Crafts-like ring closure and conversion of the terminal methoxy group to a bromide. The latter transformation may proceed either by direct SN2 displacement of the protonated methoxy group by bromide or by prior cleavage of the ether to an alcohol followed by the more conventional transformation. Displacement of the terminal bromine by dimethylamine completes construction of the side chain (8).
Ex 9: Reduction with red phosphorus and hydroiotic acid gives mostly the trans isomer of fluotracen (9).
See also
- Litracen
- Melitracen
- Danitracen
References
- Annual Reports in Medicinal Chemistry: v. 14. Academic Press Inc.,U.S. 1979. ISBN 0-12-040514-8.
- Fowler PJ, Zirkle CL, Macko E, et al. (1977). "Fluotracen: a tricyclic compound with the combined properties of antidepressants and antipsychotics in animals". Arzneimittel-Forschung. 27 (8): 1589–95. PMID 410422.
- David J. Triggle (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. ISBN 0-412-46630-9.
- Feldmann, H. S., Denber, H. C. B. (January 1980). "Fluotracen (SKF 28, 175): A new antidepressant. Double-blind study with amitriptyline". Progress in Neuro-Psychopharmacology. 4 (1): 51–55. doi:10.1016/0364-7722(80)90061-2. ISSN 0364-7722.
- Paul Norman Craig & Charles Leon Zirkle, GB 1160218 (1969 to Smith, Kline & French).
- Paul N Craig, Charles L Zirkle, U.S. Patent 3,622,630 (1971 to Smith Kline French Lab).
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
Adrenergic receptor modulators | |||||
|---|---|---|---|---|---|
| α1 |
| ||||
| α2 |
| ||||
| β |
| ||||
| |||||
Acetylcholine receptor modulators | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
Histamine receptor modulators | |||||
|---|---|---|---|---|---|
| H1 |
| ||||
| H2 |
| ||||
| H3 |
| ||||
| H4 |
| ||||
| |||||
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Classes |
|
|---|---|
| Antidepressants (TCAs and TeCAs) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Others |
|